Ammonia - hydrogen nitride - is one of the most important compounds of nitrogen and hydrogen. It is a gas without color, but with a pungent odor. The chemical composition reflects the formula of ammonia - NH 3 . An increase in pressure or a decrease in the temperature of a substance leads to its transformation into a colorless liquid. Gaseous ammonia and its solutions are widely used in industry and agriculture. In medicine, 10% ammonium hydroxide - ammonia is used.
The structure of the molecule. Electronic Ammonia Formula
The hydrogen nitride molecule in shape resembles a pyramid, at the base of which is nitrogen, bonded to three hydrogen atoms. The N – H bonds are strongly polarized. Nitrogen attracts a binding electron pair more strongly. Therefore, a negative charge accumulates on N atoms, and a positive charge is concentrated on hydrogen. The idea of this process is given by the model of the molecule, the electronic and structural formula of ammonia.
Hydrogen nitride is very soluble in water (700: 1 at 20 ° C). The presence of practically free protons leads to the formation of numerous hydrogen "bridges" that connect the molecules to each other. Structural features and chemical bonding also lead to the fact that ammonia easily liquefies with increasing pressure or lowering temperature (–33 ° C).
origin of name
The term "ammonia" was introduced into scientific use in 1801 at the suggestion of the Russian chemist Y. Zakharov, but the substance is familiar to mankind from ancient times. Gas with a pungent odor is released during the decay of waste products, many organic compounds, for example, proteins and urea, during the decomposition of ammonium salts. Chemistry historians believe that the substance was named after the ancient Egyptian god Amon. In North Africa there is an oasis of Siwa (Ammon). Surrounded by the Libyan desert , the ruins of the ancient city and the temple are preserved, next to which there are deposits of ammonium chloride. This substance in Europe was called the "Amon salt." There is a legend that the inhabitants of the Siwa oasis sniffed salt in the temple.
Obtaining hydrogen nitride
The English physicist and chemist R. Boyle in the experiments burned manure and observed the formation of white smoke over a stick soaked in hydrochloric acid and introduced into the stream of the resulting gas. In 1774, another British chemist D. Priestley heated ammonium chloride with hydrated lime and liberated a gaseous substance. Priestley called the compound "alkaline air", because its solution showed the properties of a weak base. The Boyle experiment in which ammonia interacted with hydrochloric acid was explained. Solid white ammonium chloride occurs when reacting molecules come into contact directly in the air.
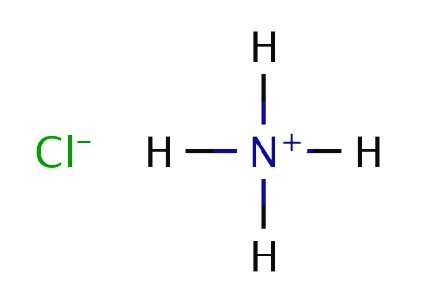
The chemical formula of ammonia was established in 1875 by the Frenchman K. Bertollet, who conducted an experiment on the decomposition of a substance into components under the influence of an electric discharge. Until now, Priestley, Boyle and Bertolle's experiments have been replicated in laboratories for the production of hydrogen nitride and ammonium chloride. The industrial method was developed in 1901 by A. Le Chatelier, who received a patent for a method for the synthesis of substances from nitrogen and hydrogen.
Ammonia solution. Formula and Properties
An aqueous solution of ammonia is usually written in the form of hydroxide - NH 4 OH. It exhibits the properties of weak alkali:
- dissociates into ions NH 3 + H 2 O = NH 4 OH = NH 4 + + OH - ;
- stains a solution of phenolphthalein in raspberry color;
- interacts with acids to form salt and water;
- precipitates Cu (OH) 2 as a bright blue substance when mixed with soluble copper salts.
The equilibrium in the reaction of the interaction of ammonia with water is shifted towards the starting substances. Preheated hydrogen nitride burns well in oxygen. Nitrogen oxidizes to diatomic molecules of the simple substance N2. Ammonia also exhibits reducing properties in a reaction with copper (II) oxide.
The value of ammonia and its solutions
Hydrogen nitride is used in the production of ammonium salts and nitric acid - one of the most important products of the chemical industry. Ammonia serves as a raw material for soda production (nitrate method). The content of hydrogen nitride in an industrial concentrated solution reaches 25%. In agriculture, an aqueous solution of ammonia is used. The formula for liquid fertilizer is NH 4 OH. The substance is directly used as a top dressing. Other methods of enriching the soil with nitrogen are the use of ammonium salts : nitrates, chlorides, phosphates. In industrial conditions and agricultural premises, it is not recommended to store mineral fertilizers containing ammonium salts with alkalis together. If the integrity of the packaging is violated, substances can react with each other with the formation of ammonia and its release into the air of the premises. A toxic compound adversely affects the respiratory system, the central nervous system of a person. A mixture of ammonia and air is explosive.