It would be useful to start by defining the concept of alkanes. These are saturated or saturated hydrocarbons, paraffins. You can also say that these are carbons in which the connection of C atoms is carried out through simple bonds. The general formula is: CnH₂n + 2.
It is known that the ratio of the number of H and C atoms in their molecules is maximum when compared with other classes. Due to the fact that all valencies are occupied either by C or H, the chemical properties of alkanes are not pronounced enough, so their second name is the phrase saturated or saturated hydrocarbons.
There is also an older name that best reflects their relative chemical inertness - paraffins, which means "deprived of affinity."
So, the topic of our conversation today: "Alkanes: homologous series, nomenclature, structure, isomerism." Data will also be provided regarding their physical properties.
Alkanes: structure, nomenclature
In them, C atoms are in a state such as sp3 hybridization. In this regard, the alkane molecule can be demonstrated as a set of tetrahedral structures of C, which are associated not only with each other, but also with H.
Between the C and H atoms there are strong, very slightly polar s-bonds. Atoms, however, around simple bonds always rotate, which is why alkane molecules take various forms, with the bond length and the angle between them being constant. Forms that are transformed into each other due to the rotation of the molecule occurring around the σ-bonds are called its conformations.
In the process of detachment of the H atom from the molecule under consideration, 1-valence particles called hydrocarbon radicals are formed. They appear as a result of compounds not only of organic substances, but also of inorganic ones. If we take away 2 hydrogen atoms from a molecule of a saturated hydrocarbon, we get 2-valent radicals.
Thus, the nomenclature of alkanes can be:
- radial (old version);
- substitution (international, systematic). It is proposed by IUPAC.
Features of radial nomenclature
In the first case, the nomenclature of alkanes is characterized by the following:
- Consideration of hydrocarbons as derivatives of methane, in which 1 or more H atoms are substituted by radicals.
- A high degree of convenience in the case of not very complex compounds.
Features of the replacement nomenclature
The substitution nomenclature of alkanes has the following features:
- The basis for the name is 1 carbon chain, while the remaining molecular fragments are considered as substituents.
- If there are several identical radicals, a number is indicated before their name (strictly in words), and radical numbers are separated by commas.
Chemistry: Nomenclature of alkanes
For convenience, the information is presented in tabular form.
Substance name | Name basis (root) | Molecular formula | The name of the carbon Deputy | Carbon Deputy Formula |
Methane | Met | Ch₄ | Methyl | Ch₃ |
Ethane | Et | C₂h₆ | Ethyl | C₂h₅ |
Propane | Prop | C₃h₈ | Propyl | C₃h₇ |
Butane | Booth | C₄h₁₀ | Butyl | C₄h₉ |
Pentane | Pent | C₅h₁₂ | Pentyl | C₅h₁₁ |
Hexane | Hex | C₆h₁₄ | Hexyl | C₆h₁₃ |
Heptane | Hept | C₇h₁₆ | Heptyl | C₇h₁₅ |
Octane | Oct | C₈h₁₈ | Octyl | C₈h₁₇ |
Nonan | Non- | C₉h₂₀ | Nonil | C₉h₁₉ |
Dean | Dec | C₁₀h₂₂ | Decile | C₁₀h₂₁ |
The above nomenclature of alkanes includes names that have developed historically (the first 4 members of a number of saturated hydrocarbons).
The names of non-expanded alkanes with 5 or more C atoms are derived from Greek numerals, which reflect a given number of C atoms. Thus, the suffix -an indicates that the substance is from a number of saturated compounds.
When compiling the names of expanded alkanes, the role of the main chain is chosen that contains the maximum number of C atoms. It is numbered so that the substituents are with the lowest number. In the case of two or more chains of the same length, the one that contains the largest number of substituents becomes the main one.
Isomerism of alkanes
Methane CH₄ acts as the parent hydrocarbon of their series. With each subsequent representative of the methane series, there is a difference from the previous one to the methylene group - CH₂. This pattern can be seen in the whole series of alkanes.
The German scientist Schiel proposed to call this series homologous. Translated from Greek means "similar, similar."
Thus, the homologous series is a set of related organic compounds having the same structure with similar chemical properties. Homologists are members of this series. Homological difference is a methylene group, into which 2 neighboring homologues differ.
As mentioned earlier, the composition of any saturated hydrocarbon can be expressed by the general formula CnH₂n + 2. Thus, the next member of the homologous series is ethane - C₂H₆. In order to deduce its structure from methane, it is necessary to replace 1 H atom with CH (figure below).
The structure of each subsequent homologue can be deduced from the previous one in the same way. As a result, propane is formed from ethane - C₃H₈.
What are isomers?
These are substances that have identical qualitative and quantitative molecular composition (identical molecular formula), however, different chemical structure, as well as having different chemical properties.
The above hydrocarbons differ in such a parameter as the boiling point: -0.5 ° - butane, -10 ° - isobutane. This type of isomerism is referred to as the isomerism of the carbon skeleton, it refers to the structural type.
The number of structural isomers is growing rapidly with an increase in the number of carbon atoms. Thus, C₁₀H₂₂ will correspond to 75 isomers (not including spatial), while for C₁₅H₃₂ 4347 isomers are already known, for C₂₀H₄₂ - 366 319.
So, it has already become clear what alkanes are, homologous series, isomerism, nomenclature. Now it’s worth moving on to the rules for compiling names according to IUPAC.
IUPAC nomenclature: rules for the formation of names
First, it is necessary to find the carbon chain in the hydrocarbon structure, which is the longest and contains the maximum number of substituents. Then you need to number the atoms of the C chain, starting from the end to which the substituent is closest.
Secondly, the basis is the name of an unbranched saturated hydrocarbon to which the most main chain corresponds by the number of C atoms.
Thirdly, before the base, it is necessary to indicate the numbers of locants near which the substituents are located. The names of substituents are written after them with a hyphen.
Fourth, in the case of identical substituents at different C atoms, the locants are combined, and a multiplication prefix appears in front of the name: di - for two identical substituents, three - for three, tetra - four, penta - for five, etc. Figures must be separated by a comma, and by a hyphen from words.
If the same C atom contains two substituents at once, the locant is also written twice.
According to these rules, the international nomenclature of alkanes is formed.
Newman's projections
This American scientist proposed special projection formulas — Newman projections — to graphically demonstrate conformations. They correspond to forms A and B and are presented in the figure below.
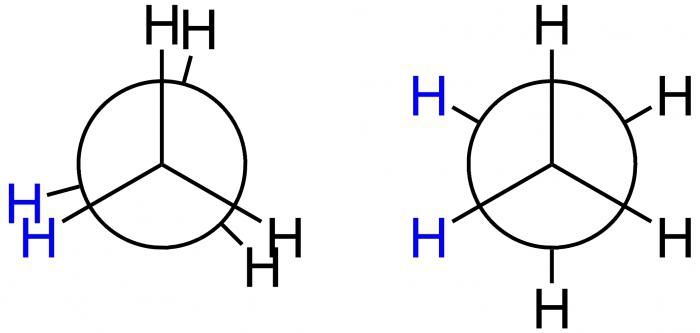
In the first case, this is an A-obscured conformation, and in the second, B-inhibited. At position A, H atoms are located at a minimum distance from each other. This form corresponds to the highest value of energy, since the repulsion between them is greatest. This is an energetically unfavorable state, as a result of which the molecule tends to leave it and move to a more stable position B. Here, the H atoms are as far apart as possible. So, the energy difference of these positions is 12 kJ / mol, due to which the free rotation around the axis in the ethane molecule, which connects the methyl groups, is uneven. After getting into an energetically advantageous position, the molecule is delayed there, in other words, it is “inhibited”. That is why it is called inhibited. The result - 10 thousand ethane molecules are in a inhibited form of conformation under the condition of room temperature. Only one has a different form - obscured.
Production of saturated hydrocarbons
It has already become known from the article that these are alkanes (their structure, nomenclature are described in detail earlier). It would be useful to consider how to obtain them. They stand out from such natural sources as oil, natural gas, associated gas, coal. Synthetic methods are also used. For example, H₂ 2H₂:
- The process of hydrogenation of unsaturated hydrocarbons: CnH₂n (alkenes) → CnH₂n + 2 (alkanes) ← CnH₂n-2 (alkynes).
- From a mixture of monoxide C and H - synthesis gas: nCO + (2n + 1) H₂ → CnH₂n + 2 + nH₂O.
- From carboxylic acids (their salts): electrolysis at the anode, at the cathode:
- Kolbe electrolysis: 2RCOONa + 2H₂O → R-R + 2CO₂ + H₂ + 2NaOH;
- Dumas reaction (alloy with alkali): CH₃COONa + NaOH (t) → CH₄ + Na₂CO₃.
- Oil cracking: CnH₂n + 2 (450-700 °) → CmH₂m + 2 + Cn-mH₂ (nm).
- Gasification of fuel (solid): C + 2H₂ → CH₄.
- Synthesis of complex alkanes (halogen derivatives) that have fewer C atoms: 2CH₃Cl (chloromethane) + 2Na → CH₃-CH₃ (ethane) + 2NaCl.
- Water decomposition of methanides (metal carbides): Al₄C₃ + 12H₂O → 4Al (OH₃) ↓ + 3CH₄ ↑.
Physical properties of saturated hydrocarbons
For convenience, the data is grouped in a table.
Formula | Alkan | Melting point in ° C | Boiling point in ° | Density, g / ml |
Ch₄ | Methane | -183 | -162 | 0.415 at t = -165 ° |
C₂h₆ | Ethane | -183 | -88 | 0.561 at t = -100 ° C |
C₃h₈ | Propane | -188 | -42 | 0.583 at t = -45 ° C |
n-C₄H₁₀ | n-butane | -139 | -0.5 | 0.579 at t = 0 ° C |
| 2-methylpropane | - 160 | - 12 | 0.557 at t = -25 ° C |
| 2,2-dimethyl propane | - 16 | 9.5 | 0.613 |
n-C₅H₁₂ | n-Pentane | -130 | 36 | 0.626 |
| 2-methylbutane | - 160 | 28 | 0.620 |
n-C₆H₁₄ | n-hexane | - 95 | 69 | 0.660 |
| 2-methylpentane | - 153 | 62 | 0.683 |
n-C₇H₁₆ | n-heptane | - 91 | 98 | 0.683 |
n-C₈H₁₈ | n-octane | - 57 | 126 | 0.702 |
| 2,2,3,3-tetra-methylbutane | - one hundred | 106 | 0.656 |
| 2,2,4-trimethyl pentane | - 107 | 99 | 0.692 |
n-C₉H₂₀ | n-nonan | - 53 | 151 | 0.718 |
n-C₁₀H₂₂ | n-dean | - thirty | 174 | 0.730 |
n-C₁₁H₂₄ | n-undecan | - 26 | 196 | 0.740 |
n-C₁₂H₂₆ | n-dodecan | - 10 | 216 | 0.748 |
n-C₁₃H₂₈ | n-Tridecan | - 5 | 235 | 0.756 |
n-C₁₄H₃₀ | n-tetradecane | 6 | 254 | 0.762 |
n-C₁₅H₃₂ | n-Pentadecane | 10 | 271 | 0.768 |
n-C₁₆H₃₄ | n-hexadecane | eighteen | 287 | 0.776 |
n-C₂₀H₄₂ | n-eicosan | 37 | 343 | 0.788 |
n-C₃₀H₆₂ | n-Triacontan | 66 | 235 at 1 mmHg st | 0.779 |
n-C₄₀H₈₂ | n-tetracontan | 81 | 260 at 3 mmHg Art. | |
n-C₅₀H₁₀₂ | n-Pentacontan | 92 | 420 at 15 mmHg Art. | |
n-C₆₀H₁₂₂ | n-hexacontane | 99 | | |
n-C₇₀H₁₄₂ | n-Heptacontan | 105 | | |
n-C₁₀₀H₂₀₂ | n-hectane | 115 | | |
Conclusion
The article considered such a concept as alkanes (structure, nomenclature, isomerism, homologous series, etc.). Little is told about the features of radial and replacement nomenclatures. Methods for producing alkanes are described.
In addition, the article lists in detail the entire nomenclature of alkanes (a test can help to absorb the information received).