The need to use mechanical energy in production has led to the emergence of heat engines.
The device of heat engines
A heat engine (heat engine) is a device for converting internal energy into mechanical energy.
Any heat engine has a heater, a working fluid (gas or steam), which, as a result of heating, does the work (drives the turbine shaft, moves the piston, and so on) and a refrigerator. The figure below shows a diagram of a heat engine.
The basics of the action of heat engines
Each heat engine is powered by an engine. To do the job, he needs to have a pressure difference on either side of the engine piston or turbine blades. This difference is achieved in all heat engines as follows: the temperature of the working fluid rises by hundreds or thousands of degrees in comparison with the ambient temperature. In gas turbines and internal combustion engines (ICE), the temperature rises due to the fact that the fuel burns inside the engine itself. The refrigerator may be an atmosphere or a special purpose device for condensing and cooling exhaust steam.
Carnot cycle
A cycle (a circular process) is a set of changes in the state of a gas, as a result of which it returns to its original state (can do work). In 1824, the French physicist Sadi Carnot showed that the heat engine cycle (Carnot cycle), which consists of two processes, isothermal and adiabatic, is beneficial. The figure below shows a graph of the Carnot cycle: 1-2 and 3-4 - isotherms, 2-3 and 4-1 - adiabats.
In accordance with the law of conservation of energy, the operation of thermal machines, which the engine performs, is equal to:
A = Q 1 - Q 2 ,
where Q 1 is the amount of heat that is received from the heater, and Q 2 is the amount of heat that is devoted to the refrigerator.
The efficiency of a heat engine is the ratio of the work A that the engine performs to the amount of heat that is received from the heater:
η = A / Q = (Q 1 - Q 2 ) / Q 1 = 1 - Q 2 / Q 1 .
In the work "Thoughts on the driving force of fire and on machines that are capable of developing this force" (1824) Carnot described a heat engine called "an ideal heat engine with an ideal gas, which is a working fluid." Thanks to the laws of thermodynamics, it is possible to calculate the efficiency (maximum possible) of a heat engine with a heater that has a temperature of T 1 and a refrigerator with a temperature of T 2 . The Carnot heat engine has an efficiency of:
η max = (T 1 - T 2 ) / T 1 = 1 - T 2 / T 1.
Sadi Carnot proved that any kind of heat engine is real, which works with a heater with a temperature of T 1 and a refrigerator with a temperature of T 2 is not able to have an efficiency that would exceed the efficiency of a heat engine (ideal).
Internal combustion engine (ICE)
A four-stroke ICE consists of one or more cylinders, a piston, a crank mechanism, intake and exhaust valves, and candles.
The duty cycle consists of four clock cycles:
1) suction - the combustible mixture enters the cylinder through the valve;
2) compression - both valves are closed;
3) working stroke - explosive combustion of a combustible mixture;
4) exhaust - the release of exhaust gases into the atmosphere.
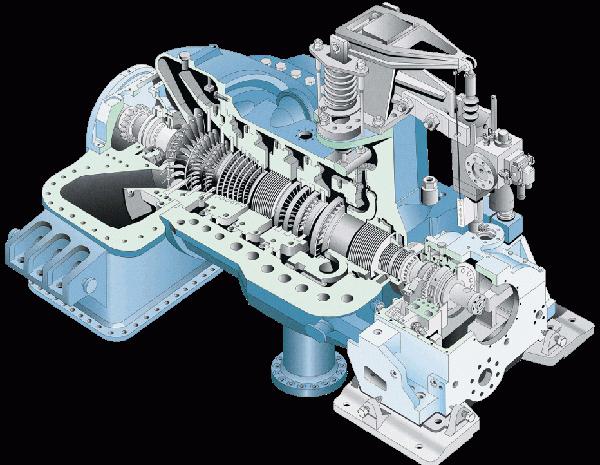
Steam turbine
In a steam turbine, energy conversion occurs due to the difference in pressure of water vapor at the inlet and outlet.
The capacity of modern steam turbines reaches 1300 MW.
Some technical parameters of a 1200 MW steam turbine
- The vapor pressure (fresh) is 23.5 MPa.
- Steam temperature - 540 ° .
- Turbine steam consumption - 3600 t / h.
- The rotor speed is 3000 rpm.
- The vapor pressure in the condenser is 3.6 kPa.
- The length of the turbine is 47.9 m.
- The mass of the turbine is 1900 tons.
The heat engine consists of an air compressor, a combustion chamber and a gas turbine. Principle of operation: the air is adiabatically sucked into the compressor, so its temperature rises to 200 ° C or more. Then, compressed air enters the combustion chamber, where simultaneously under high pressure liquid fuel enters - kerosene, photogen, fuel oil. When burning fuel, the air heats up to a temperature of 1500-2000 ° C, expands, and its speed increases. Air moves at high speed, and combustion products are sent to the turbine. After the transition from stage to stage, the combustion products give their kinetic energy to the turbine blades. Part of the energy received by the turbine goes to the rotation of the compressor; the rest is spent on the rotation of the rotor of the generator, the propeller of an airplane or a sea vessel, the wheels of a car.
A gas turbine can be used, in addition to rotating the wheels of a car and the propellers of an airplane or motor ship, as a jet engine. Air and combustion products are ejected from the gas turbine at high speed, therefore, the jet thrust that occurs during this process can be used for the movement of air (aircraft) and water (ship) ships, railway transport. For example, turboprop engines have the An-24, An-124 (Ruslan), An-225 (Dream) aircraft. So, “Dream” at a flight speed of 700-850 km / h is capable of transporting 250 tons of cargo to a distance of almost 15,000 km. It is the largest transport aircraft in the world.
Ecological problems of heat engines
A great influence on the climate has a state of the atmosphere, in particular the presence of carbon dioxide and water vapor. Thus, a change in the carbon dioxide content leads to an increase or weakening of the greenhouse effect, in which carbon dioxide partially absorbs the heat that the Earth radiates into space, traps it in the atmosphere and thereby increases the temperature of the surface and lower layers of the atmosphere. The greenhouse effect plays a crucial role in mitigating the climate. In its absence, the average temperature of the planet would not be +15 ° , but lower by 30-40 ° .
Now in the world there are more than 300 million different types of cars that create more than half of all air pollution.
Over 1 year, 150 million tons of sulfur oxides, 50 million tons of nitric oxide, 50 million tons of ash, 200 million tons of carbon monoxide, 3 million tons of pheon are emitted from thermal power plants as a result of fuel combustion.
The atmosphere includes ozone, which protects all life on earth from the harmful effects of ultraviolet rays. In 1982, J. Farman, an English researcher, discovered an ozone hole over Antarctica - a temporary decrease in atmospheric ozone. At the time of maximum development of the ozone hole on October 7, 1987, the amount of ozone in it decreased by 2 times. The ozone hole probably arose as a result of anthropogenic factors, including the use in industry of chlorine-containing freons (freons) that destroy the ozone layer. However, studies in 1990. did not confirm this view. Most likely, the appearance of the ozone hole is not related to human activity and is a natural process. In 1992, an ozone hole was discovered over the Arctic.
If all atmospheric ozone is collected in a layer near the surface of the Earth and thickened to air density at normal atmospheric pressure and a temperature of 0 ° C, then the thickness of the ozone shield will be only 2-3 mm! That's the whole shield.
A bit of history ...
- July 1769 In the Parisian park of Meudon, a military engineer N.J. Cunillot traveled several tens of meters on a "fire cart", which was equipped with a two-cylinder steam engine.
- 1885 year. Karl Benz, a German engineer, built the first 0.66 kW Motorwagen four-stroke gasoline three-wheeled vehicle, for which he received a patent on January 29, 1886. The speed of the car reached 15-18 km / h.
- 1891 year. Gottlieb Daimler, a German inventor, manufactured a truck with a 2.9 kW (4 horsepower) engine from a passenger car. The maximum speed of the car reached 10 km / h, the carrying capacity in various models ranged from 2 to 5 tons.
- 1899 year. The Belgian K. Zhenatzi for the first time overcame the 100-kilometer speed limit in his Jame Contant car (Always Displeased).
Examples of solving problems
Task 1. The temperature of the heater of an ideal heat engine is 2000 K, and the temperature of the refrigerator is 100 ° C. Determine the efficiency.
Solution :
The formula that determines the efficiency of the heat engine (maximum):
ŋ = T 1 -T 2 / T 1.
ŋ = (2000K - 373K) / 2000 K = 0.81.
Answer: engine efficiency is 81%.
Task 2 In a heat engine, when burning fuel, 200 kJ of heat was obtained, and 120 kJ of heat was transferred to the refrigerator. What is the engine efficiency?
Decision:
The formula for determining the efficiency is as follows:
ŋ = Q1 - Q2 / Q1.
ŋ = (2 · 10 5 J - 1.2 · 10 5 J) / 2 · 10 5 J = 0.4.
Answer: the efficiency of the heat engine is 40%.
Task 3. What is the efficiency of the heat engine if the working fluid after receiving the amount of heat 1.6 MJ from the heater has completed 400 kJ? How much heat was transferred to the refrigerator?
Decision:
Efficiency can be determined by the formula
ŋ = A / Q 1.
ŋ = 0.4 · 10 6 J / 1.6 · 10 6 J = 0.25.
The amount of heat transferred to the refrigerator can be determined by the formula
Q 1 - A = Q 2.
Q 2 = 1.6 · 10 6 J - 0.4 · 10 6 J = 1.2 · 10 6 J.
Answer: the heat engine has an efficiency of 25%; the amount of heat transferred to the refrigerator is 1.2 · 10 6 J.