Diluted and concentrated sulfuric acid is such an important chemical product that more than any other substance is produced in the world. The economic wealth of a country can be estimated by the volume of sulfuric acid produced in it.
Dissociation process
Sulfuric acid is used in the form of aqueous solutions of various concentrations. It undergoes a dissociation reaction in two stages, producing H + ions in solution.
H 2 SO 4 = H + + HSO 4 - ;
HSO 4 - = H + + SO 4 -2 .
Sulfuric acid is strong, and the first stage of its dissociation is so intense that almost all of the starting molecules decompose into H + ions and HSO 4 -1 ions (hydrosulfate) in solution. The latter partially decompose further, releasing another H + ion and leaving a sulfate ion (SO 4 -2 ) in solution. However, hydrosulfate, being a weak acid, still prevails in solution over H + and SO 4 -2 . Its complete dissociation occurs only when the density of the sulfuric acid solution approaches the density of water, i.e., with strong dilution.
Properties of sulfuric acid
It is special in the sense that it can act as a regular acid or as a strong oxidizing agent, depending on its temperature and concentration. A cold dilute solution of sulfuric acid reacts with active metals to produce salt (sulfate) and hydrogen gas. For example, the reaction between cold diluted H 2 SO 4 (assuming its complete two-stage dissociation) and metallic zinc looks like this:
Zn + H 2 SO 4 = ZnSO 4 + H 2 .
Concentrated hot sulfuric acid, whose density is about 1.8 g / cm 3 , can act as an oxidizing agent, reacting with materials that are usually inert to acids, such as, for example, metallic copper. During the reaction, copper oxidizes, and the acid mass decreases, a solution of copper (II) sulfate in water and gaseous sulfur dioxide (SO 2 ) instead of hydrogen are formed, which could be expected when the acid reacted with the metal.
Cu + 2H 2 SO 4 = CuSO 4 + SO 2 + 2H 2 O.
How is the concentration of solutions expressed?
Actually, the concentration of any solution can be expressed in various ways, but the most widely used weight concentration. It shows the number of grams of solute in a certain mass or volume of a solution or solvent (usually 1000 g, 1000 cm 3 , 100 cm 3 and 1 dm 3 ). Instead of the mass of the substance in grams, you can take its amount, expressed in moles, - then the molar concentration is obtained per 1000 g or 1 dm 3 of solution.
If the molar concentration is determined in relation not to the quantity of the solution, but only to the solvent, then it is called the molality of the solution. It is characterized by independence from temperature.
Often, the weight concentration is indicated in grams per 100 g of solvent. Multiplying this indicator by 100%, get it in weight percent (percentage concentration). This method is the most commonly used as applied to sulfuric acid solutions.
Each value of the solution concentration determined at a given temperature corresponds to its specific density (for example, the density of a solution of sulfuric acid). Therefore, sometimes a solution is characterized by it. For example, a solution of H 2 SO 4 , characterized by a percentage concentration of 95.72%, has a density of 1.835 g / cm 3 at t = 20 ° C. How to determine the concentration of such a solution, if only the density of sulfuric acid is given? A table giving such a correspondence is an integral part of any textbook on general or analytical chemistry.
Concentration Example
Let's try to move from one way of expressing the concentration of a solution to another. Suppose we have a solution of H 2 SO 4 in water with a percentage concentration of 60%. First, determine the appropriate density of sulfuric acid. A table containing percentages (first column) and their corresponding densities of an aqueous solution of H 2 SO 4 (fourth column) is shown below.
Using it, we determine the desired value, which is equal to 1.4987 g / cm 3 . We now calculate the molarity of this solution. To do this, determine the mass of H 2 SO 4 in 1 liter of solution and the corresponding number of moles of acid.
Volume occupied by 100 g of the initial solution:
100 / 1.4987 = 66.7 ml.
Since 66.7 milliliters of a 60% solution contains 60 g of acid, 1 l of it will contain:
(60 / 66.7) x 1000 = 899, 55 g.
The molar weight of sulfuric acid is 98. Hence, the number of moles contained in 899.55 g of its grams will be equal to:
899.55 / 98 = 9.18 mol.
The concentration dependence of sulfuric acid is shown in Fig. below.
The use of sulfuric acid
It is used in various industries. In the production of pig iron and steel, it is used to clean the surface of a metal before it is coated with another substance, and is involved in the creation of synthetic dyes, as well as other types of acids, such as hydrochloric and nitric. It is also used in the manufacture of pharmaceuticals, fertilizers and explosives, and is also an important reagent for the removal of impurities from oil in the oil refining industry.
This chemical is incredibly useful in everyday life, and is easily available as a solution of sulfuric acid used in lead-acid batteries (for example, those that are in cars). Such an acid, as a rule, has a concentration of from about 30% to 35% H 2 SO 4 by weight, the rest is water.
For many domestic applications, 30% H 2 SO 4 will be more than enough to satisfy your needs. However, a much higher concentration of sulfuric acid is also required in industry. Usually in the production process it is first obtained sufficiently diluted and contaminated with organic impurities. Concentrated acid is obtained in two stages: first it is brought up to 70%, and then - at the second stage - it is raised to 96-98%, which is the ultimate indicator for economically viable production.
Density of sulfuric acid and its varieties
Although almost 99% sulfuric acid can be obtained briefly by boiling, the subsequent loss of SO 3 at the boiling point leads to a decrease in concentration to 98.3%. In general, a species with an index of 98% is more stable in storage.
Commercial varieties of acid differ in its percentage concentration, and those values are selected for them at which crystallization temperatures are minimal. This is done to reduce the precipitation of sulfuric acid crystals during transportation and storage. The main varieties are as follows:
- Tower (nitrous) - 75%. The density of sulfuric acid of this grade is 1670 kg / m 3 . Get it so-called. by the nitrous method, in which the calcining gas obtained during the firing of primary raw materials containing sulfur dioxide SO 2 is treated with nitrosa in the lined towers (hence the name of the variety) (this is also H 2 SO 4 , but with nitrogen oxides dissolved in it). As a result, acid and nitrogen oxides are released, which are not consumed in the process, but are returned to the production cycle.
- Contact - 92.5-98.0%. The density of sulfuric acid of 98% of this grade is 1836.5 kg / m 3 . It is also obtained from a calcining gas containing SO 2 , the process involving the oxidation of dioxide to SO 3 anhydride upon its contact (hence the name of the variety) with several layers of a solid vanadium catalyst.
- Oleum - 104.5%. Its density is 1896.8 kg / m 3 . This is a solution of SO 3 in H 2 SO 4 , in which the first component contains 20%, and the acid is exactly 104.5%.
- High percentage oleum - 114.6% . Its density is 2002 kg / m 3 .
- Rechargeable - 92-94%.
How is a car battery
The work of this one of the most popular electrical devices is entirely based on electrochemical processes that occur in the presence of an aqueous solution of sulfuric acid.
A car battery contains diluted sulfuric acid electrolyte, as well as positive and negative electrodes in the form of several plates. Positive plates are made of a reddish-brown material - lead dioxide (PbO 2 ), and negative ones are made of grayish "spongy" lead (Pb).
Since the electrodes are made of lead or a lead-containing material, this type of battery is often called a lead-acid battery. Its operability, i.e., the magnitude of the output voltage, is directly determined by the current density of sulfuric acid (kg / m3 or g / cm 3 ), which is poured into the battery as an electrolyte.
What happens to the electrolyte when the battery is low
The lead-acid battery electrolyte is a solution of battery sulfuric acid in chemically pure distilled water with a percentage concentration of 30% when fully charged. Pure acid has a density of 1.835 g / cm 3 , the electrolyte is about 1,300 g / cm 3 . When the battery is discharged, electrochemical reactions occur in it, as a result of which sulfuric acid is taken from the electrolyte. The density depends on the concentration of the solution almost proportionally, therefore, it should decrease due to a decrease in the concentration of electrolyte.
As long as the discharge current flows through the battery, the acid near its electrodes is actively used, and the electrolyte becomes more diluted. Diffusion of acid from the volume of the entire electrolyte and to the electrode plates maintains an approximately constant intensity of chemical reactions and, as a consequence, the output voltage.
At the beginning of the discharge process, the diffusion of acid from the electrolyte into the plates occurs quickly because the sulfate formed in this case has not clogged the pores in the active material of the electrodes. When sulfate begins to form and fill the pores of the electrodes, diffusion occurs more slowly.
It is theoretically possible to continue the discharge until all the acid has been consumed and the electrolyte consists of pure water. However, experience shows that discharges should not continue after the electrolyte density has dropped to 1,150 g / cm 3 .
When the density drops from 1,300 to 1,150, this means that so much sulfate was formed during the reactions and it fills all the pores in the active materials on the plates, i.e. almost all sulfuric acid has already been taken from the solution. Density depends on concentration proportionally, and the battery charge also depends on density. In fig. below shows the dependence of the battery charge on the density of the electrolyte.
Changing the density of the electrolyte is the best way to determine the state of discharge of the battery, provided that it is used properly.
The degree of discharge of the car battery depending on the density of the electrolyte
Its density should be measured every two weeks, and a record of indications for future use should be kept constantly.
The denser the electrolyte, the more acid it contains, and the more charged the battery. A density of 1,300-1,280 g / cm 3 indicates a full charge. As a rule, the following degrees of battery discharge differ depending on the density of the electrolyte:
- 1,300-1,280 - fully charged:
- 1,280-1,200 - more than half discharged;
- 1,200-1,150 - less than half charged;
- 1,150 - almost empty.
For a fully charged battery, before connecting its car network, the voltage of each cell is from 2.5 to 2.7 V. As soon as the load is connected, the voltage quickly drops to about 2.1 V in three or four minutes. This is due to the formation of a thin layer of lead sulfate on the surface of the negative electrode plates and between the layer of lead peroxide and the metal of the positive plates. The final value of the cell voltage after connecting to the car network is about 2.15-2.18 volts.
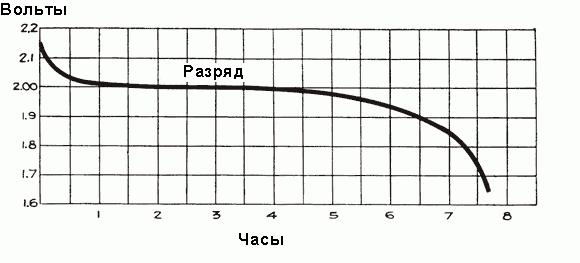
When the current begins to flow through the battery during the first hour of operation, a voltage drop of up to 2 V occurs, due to an increase in the internal resistance of the cells due to the formation of more sulfate, which fills the pores of the plates, and the selection of acid from the electrolyte. Shortly before the start of the current flow , the electrolyte density is maximum and equal to 1,300 g / cm 3 . At first, its rarefaction occurs quickly, but then a balanced state is established between the acid density near the plates and in the main volume of the electrolyte, acid selection by electrodes is supported by the arrival of new parts of the acid from the main part of the electrolyte. In this case, the average density of the electrolyte continues to decrease steadily according to the dependence shown in Fig. above. After the initial drop, the voltage decreases more slowly, the rate of decrease depends on the load of the battery. The timeline of the discharge process is shown in Fig. below.
Monitoring the state of the electrolyte in the battery
A density meter is used to determine the density. It consists of a small sealed glass tube with an extension at the lower end filled with shot or mercury, and a graduated scale at the upper end. This scale is labeled from 1,100 to 1,300 with various intermediate values, as shown in fig. below. If this hydrometer is placed in an electrolyte, then it will sink to a certain depth. In this case, it will displace a certain volume of electrolyte, and when an equilibrium position is reached, the weight of the displaced volume will simply be equal to the weight of the hydrometer. Since the density of the electrolyte is equal to the ratio of its weight to volume, and the weight of the hydrometer is known, each level of immersion in the solution corresponds to a specific density.
Some hydrometers do not have a scale with density values, but are marked with the words: “Charged”, “Half Discharge”, “Full Discharge” or the like.