The simplest polyhydric alcohol, in which there are 3 OH groups, is glycerol. The formula common to compounds of this type is CnH2n - 1 (OH) 3. In order to better understand the properties and application of glycerol and its homologues, we consider varieties of formulas of the substance, each of which is used in certain situations.
Classification and nomenclature of glycerins
In organic chemistry, alcohols are substances derived from hydrocarbons. Part of the hydrogen atoms in the molecules is substituted by one or more hydroxy groups. Alcohols differ in the number of OH groups (mono-, di-, polyatomic). The lower representatives of the class with the number of carbon atoms from 1 to 12 are liquid substances, the higher are solids. Alcantriols, or glycerins, are triatomic alcohols containing three hydroxyls associated with three different carbon atoms. Compounds belonging to this group exhibit amphoteric properties due to the mutual influence of the oxy group and the radical.
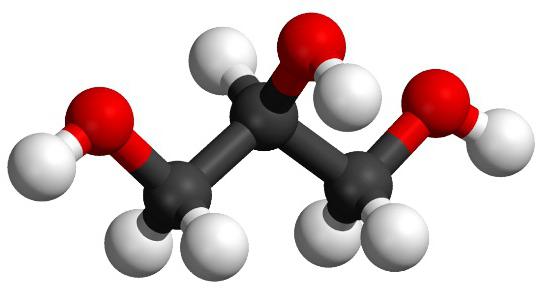
The simplest representative of alkanetriols is propanetriol-1,2,3 (a synonym is glycerin). The formula of the substance is C 3 H 8 O 3 . The systematic nomenclature involves the mention of the name of the corresponding alkane with the word "triol", the use of Arabic numerals that determine the position of the OH group. The numbering in the molecules of the glycerol homologs is from the nearest hydroxyl to the end of the chain. Possible types of isomerism: carbon chain structure, position of hydroxy groups, optical.
Discovery of glycerin
The Swedish pharmacist K. Scheele in 1779, when saponifying fats, first received a new syrup-like substance. After 33 years, the Frenchman M. Chevrel called the sweet liquid glycerin.
The chemical composition was established by Peluse in 1836. A significant contribution to the study of the structure was made by Berthelot (1854) and Wurz (1857), who continued to study glycerol. The molecular formula and nature of the radical made it possible to attribute glycerol to saturated alcohols.
The need for glycerin increased significantly after 1847, when the nitric acid ester was discovered. In 1875, the Swedish engineer A. Nobel managed to obtain explosives with dynamite, using glycerol.
The composition, structure and simplest formula of glycerin
The simplest record of the composition of the substance coincides with the true, empirical and gross formula of glycerol - C 3 H 8 O 3 . The carbon chain has 3 atoms, each of them is associated with a hydroxy group. Chemical symbols indicate the atoms that make up the substance: C - carbon, O - oxygen, H - hydrogen. The composition of glycerol is reflected in different formulas (molecular, structural). In the study of matter, ball-rod and hemispherical models are widely used. Two-dimensional and three-dimensional structures created using computer simulation are spatial images of a glycerol molecule. They allow you to visualize the composition, relative position and distance, the angle of coupling between atoms.
Molecular and Molar Masses of Glycerol
By the formula, you can find the molecular and molar masses, the percentage of elements in the substance. For calculations, it is necessary to use the atomic masses of the elements indicated in the periodic table. The empirical formula of glycerin is: C 3 H 5 (OH) 3 . By multiplying the atomic mass (in amu) of each element by the number of atoms, followed by adding up the obtained values, we find the molecular (Mr) and molar (M) masses. For that type of calculation, it is more convenient to use the gross formula of glycerol - C 3 H 8 O 3 .
- Ar (H) = 1.00794; the number of atoms in the molecule is 8.
- Ar (C) = 12.0107; atoms - 3.
- Ar (O) = 15.9994; atoms - 3.
- Mr (C 3 H 8 O 3 ) = 12.0107 * 3 + 1.00794 * 8 + 15.9994 * 3 = 92.09382 a. eat.
- M (C 3 H 8 O 3 ) = 92.09382 g / mol /
- The percentage of elements in the molecule of the substance: H - 8.756%, C - 39.125%, O - 52.119%.
The rational and structural formula of glycerin
The composition of the substance and its molecules reflect the rational and gross formula, but they do not give an idea of the arrangement of atoms, which differs glycerin. The formula structural and computer model are better suited to study the structure of the molecule, the bonds between atoms.
- The rational formula for glycerol is C 3 H 5 (OH) 3 . From the composition of the molecule, OH functional groups are selected and enclosed in parentheses. Immediately after the closing bracket, the number of oxy groups in the molecule is indicated.
- A semi-expanded form of the rational formula is HOCH 2 CH (OH) CH 2 OH (glycerin).
- The structural formula in graphical form shows the location of the molecule. Dashes between atoms symbolize chemical bonds.
- The Lewis structure contains points denoting valence electrons and pairs involved in bond formation.
Some types of images of the molecule take up a lot of space, therefore they often use abbreviated formulas, for example, HOSN 2 —CHOH — CH 2 OH, as well as skeletal structures:
The state of atoms in a glycerol molecule
Hydroxyl is a polar particle, and oxygen also has a lone pair of electrons. The presence of three hydroxy groups leads to further polarization of the O – H bond. A partial “+” charge appears on the carbon atoms, facilitating the nucleophilic substitution of hydroxyl. Features of the composition and structure, which reflects the structural formula of glycerol, are confirmed in the properties of the substance. Numerous hydrogen bridges are characteristic of this compound — weak additional bonds. Glycerin has more pronounced acid properties compared to ethanol and propanol. Among the most important derivatives is glycerol trioleate. Formula:
- the simplest - C 57 N 104 O 6 ;
- half-developed rational - ( 17 33 ) 3 3 5 ;
- rational with structural and skeletal elements -
The appearance of glycerin
At room temperature, propanetriol-1,2,3 is a colorless or pale yellow liquid, odorless, sweet in taste. Glycerin hardened at low temperatures melts at a temperature of 17.8 ° C. Boiling of the substance with subsequent evaporation begins at 290 ° C. Glycerin is slightly heavier than water; calculating its density at 20 ° C gives a value of 1.2604 g / cm3.
Properties of propanetriol-1,2,3
The chemical formula of glycerol does not give an idea of the amphoteric nature of the compound. Weak acidic and basic properties of substances are associated with the peculiarities of the influence of atoms in a molecule, polarization in the O – H group. In the presence of alkali, glycerol interacts with copper (II) hydroxide , and a blue-colored complex is obtained (one of the qualitative reactions). With acids, the reaction of glycerol ends with the formation of esters. The interaction of a trihydric alcohol with nitric acid in the presence of H 2 SO 4 (conc.) Leads to the formation of nitroglycerin.
At home, from fats and oils with the help of glycerin, ethyl alcohol, other ingredients, soap is obtained. The cooking process requires careful heating of the mass in a water bath, a creative attitude to the selection of components and forms for the finished soap product.
Glycerin and its derivatives are used in enamels, paints, many medicines, and toiletries. It contains a sweet substance in a wide variety of foods, including baked goods. The international name for sugar substitute and confectionery flavor is E422. Along with other alcohols, as well as fatty acid esters, glycerin is considered as a potential replacement for fuel derived from oil. Economical methods of using new varieties of biodiesel for refueling cars will revolutionize the global transportation industry. The environmental situation will improve significantly, the dependence of the world economy on oil and gas production will decrease.