About the anode and cathode of the power source must be known to those involved in practical electronics. What and how is it called? Why so? There will be an in-depth consideration of the topic from the point of view of not only amateur radio, but also chemistry. The most popular explanation is as follows: the anode is the positive electrode, and the cathode is the negative. Alas, this is not always true and incomplete. In order to be able to determine the anode and cathode, it is necessary to have a theoretical base and know what and how. Let's look at this in an article.
Anode
We turn to GOST 15596-82, which deals with chemical
current sources. We are interested in the information posted on the third page. According to GOST, the anode is the negative electrode of a
chemical current source . So yes! Why exactly? The fact is that it is through it that the electric current enters from the external circuit into the source itself. As you can see, not everything is as easy as it seems at first glance. You can advise you to carefully consider the pictures presented in the article, if the content seems too complicated - they will help to understand what the author wants to convey to you.
Cathode
We turn to the same GOST 15596-82. A positive electrode of a chemical current source is one when discharged from which it exits into an external circuit. As you can see, the data contained in GOST 15596-82, consider the situation from a different perspective. Therefore, when consulting with other people about certain designs, you must be very careful.
Occurrence of Terms
They were introduced by Faraday in January 1834 in order to avoid ambiguity and achieve greater accuracy. He proposed his own version of memorization using the example of the Sun. So, he has an anode - this is sunrise. The sun moves up (current enters). The cathode is a call. The sun is moving down (current is coming out).
Example of a radio tube and diode
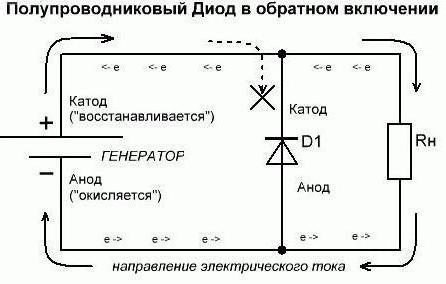
We continue to understand what is used to indicate what. Suppose we have one of these energy consumers in the open state (in direct connection). So, an electric current enters the element through the anode from the external circuit of the diode. But do not get confused by such an explanation with the direction of the electrons. An electric current exits from the element used through the cathode into the external circuit. The situation that has developed now resembles cases when people look at an inverted picture. If these notations are complex - remember that understanding them in such a way is mandatory exclusively for chemists. Now let's do the reverse inclusion. It can be noted that semiconductor diodes will practically not conduct current. The only possible exception is the reverse breakdown of the elements. And electrovacuum diodes (kenotrons, radio tubes) will not conduct reverse current at all. Therefore, it is considered (conditionally) that he does not go through them. Therefore, formally, the conclusions of the anode and cathode of the diode do not fulfill their functions.
Why is there confusion?
Specifically, in order to facilitate training and practical application, it was decided that the diode elements of the name of the conclusions will not change depending on their switching circuit, and they will be “attached” to the physical conclusions. But this does not apply to batteries. So, for semiconductor diodes, it all depends on the type of conductivity of the crystal. In electron tubes, this issue is tied to an electrode that emits electrons at the location of the filament. Of course, there are certain nuances here: for example, reverse current can flow through semiconductor devices such as a suppressor and a zener diode, but there is a specificity that clearly goes beyond the scope of this article.
We deal with the electric battery
This is a truly classic example of a chemical source of electric current that is renewable. The battery is in one of two modes: charge / discharge. In both of these cases there will be a different direction of electric current. But note that the polarity of the electrodes will not change. And they can act in different roles:
- During charging, the positive electrode receives an electric current and is the anode, while the negative electrode releases it and is called the cathode.
- If there is no movement about them, it makes no sense to talk.
- During the discharge, the positive electrode releases an electric current and is the cathode, and the negative accepts and is called the anode.
We'll put a word about electrochemistry
Here, slightly different definitions are used. So, the anode is considered as an electrode where oxidative processes occur. And recalling the school chemistry course, can you answer what happens in another part? The electrode on which the recovery processes take place is called the cathode. But there is no reference to electronic devices. Let's look at the value of redox reactions for us:
- Oxidation. There is a process of recoil by an electron particle. The neutral turns into a positive ion, and the negative is neutralized.
- Recovery. There is a process of obtaining a particle of an electron. Positive turns into a neutral ion, and then into a negative ion when repeated.
- Both processes are interconnected (so, the number of electrons that are given is equal to their attached number).
Also, for designation, Faraday introduced names for elements that take part in chemical reactions:
- Cations. So called positively charged ions that move in the electrolyte solution toward the negative pole (cathode).
- Anions This is the name of negatively charged ions that move in the electrolyte solution toward the positive pole (anode).
How do chemical reactions occur?
The oxidizing and reducing half-reactions are separated in space. The transition of electrons between the cathode and anode is not carried out directly, but thanks to the conductor of the external circuit, on which an electric current is generated. Here you can observe the mutual conversion of electrical and chemical forms of energy. Therefore, for the formation of the external circuit of the system from conductors of various kinds (which are the electrodes in the electrolyte), it is necessary to use metal. You see, the voltage between the anode and cathode exists, as does one nuance. And if there were no element that prevents them from directly producing the necessary process, the value of the sources of chemical current would be very low. And so, due to the fact that the charge needs to go through that scheme, the equipment was assembled and works.
What is what: step 1
Now let's determine what is what. Take the galvanic cell of Jacobi-Daniel. On the one hand, it consists of a zinc electrode, which is immersed in a solution of zinc sulfate. Then comes the porous septum. And on the other hand there is a copper electrode, which is located in a solution of
copper sulfate. They are in contact with each other, but the chemical characteristics and the partition do not allow to mix.
Step 2: The Process
Zinc oxidizes, and the electrons move along the external circuit to copper. It turns out that the galvanic cell has an anode charged negatively, and the cathode is positive. Moreover, this process can proceed only in those cases when the electrons have a place to "go". The fact is that getting directly from the electrode to another is prevented by the presence of “isolation”.
Step 3: Electrolysis
Let's look at the electrolysis process. Installation for its passage is a vessel in which there is a solution or molten electrolyte. Two electrodes are lowered into it. They are connected to a direct current source. The anode in this case is an electrode that is connected to the positive pole. This is where the oxidation occurs. A negatively charged electrode is the cathode. Here the recovery reaction proceeds.
Step 4: Lastly
Therefore, when operating with these concepts, it is always necessary to take into account that the anode is not used in 100% of cases to denote a negative electrode. Also, the cathode may periodically lose its positive charge. It all depends on whether the process proceeds on the electrode: reducing or oxidizing.
Conclusion
That’s how it is - it’s not very difficult, but you won’t say that it’s simple. We examined the galvanic cell, anode and cathode from the point of view of the circuit, and now you should not have problems with connecting power supplies with operating time. And finally, you need to leave some more valuable information for you. You always have to consider the difference that the cathode potential / anode potential has . The fact is that the first will always be a little big. This is due to the fact that the efficiency does not work with an indicator of 100% and part of the charges dissipate. It is because of this that you can see that batteries have a limit on the number of times a charge and discharge.