It was not the desire to drink alcohol that served as an impetus for the emergence of the alcohol industry. The first to practically solve the problem of how to separate water and alcohol were not consumers, or even potion producers, but ... doctors. Alcohol is incomparably the best solvent in comparison with water, therefore, on its basis the ancient eastern doctors successfully prepared their potions. The distillation method was brought to Europe, as one of the trophies, the knights of their crusades. In the Old World, the method took root, however, in France, received another purpose - the manufacture of cosmetics. And only then the products of haulage mash began to slowly gain a place under the sun as drinks, which is associated with many legends and traditions.
The method every grandmother knows about
The substance from which alcohol is usually obtained is mash, and moonshining is the oldest way to separate the mixture. True, the process does not provide alcohol and water in its pure form, but it is cheap, reliable and worked out for centuries.
In the process of fermentation, sugar is produced from sugar-containing substances by alcohol, and at a certain concentration they themselves die in it (alcohol, it turns out, is poison even for the yeast producing it). Then pure physics comes into play: water, as you know, at normal atmospheric pressure (about 760 mm Hg) turns into steam at a temperature of 100 ° C, but ethanol - ethanol, the very one that creates the "festive" "mood, boils at a temperature of about 78 ° C.
Thus, the way how to separate water and alcohol is obvious. When heated, ethanol vapors are the first to evaporate and are removed from the boiling zone. Then the vapor enters the cooler and condenses, turns back into liquid. Water remains in the tank.
How did you learn how to get pure alcohol?
However, in reality, not everything is so simple and easy. At 80 ° C, the alcohol evaporates vigorously and leaves the tank. Water, although it does not reach the boiling point at a given temperature, but part of it nevertheless evaporates and enters the cooler along with ethanol vapor. If distillation is done 3 or more times, the maximum strength that can be achieved is 80 ... 85%. But how to separate water and alcohol completely or almost completely? For this, at the end of the 19th century a distillation column was invented.
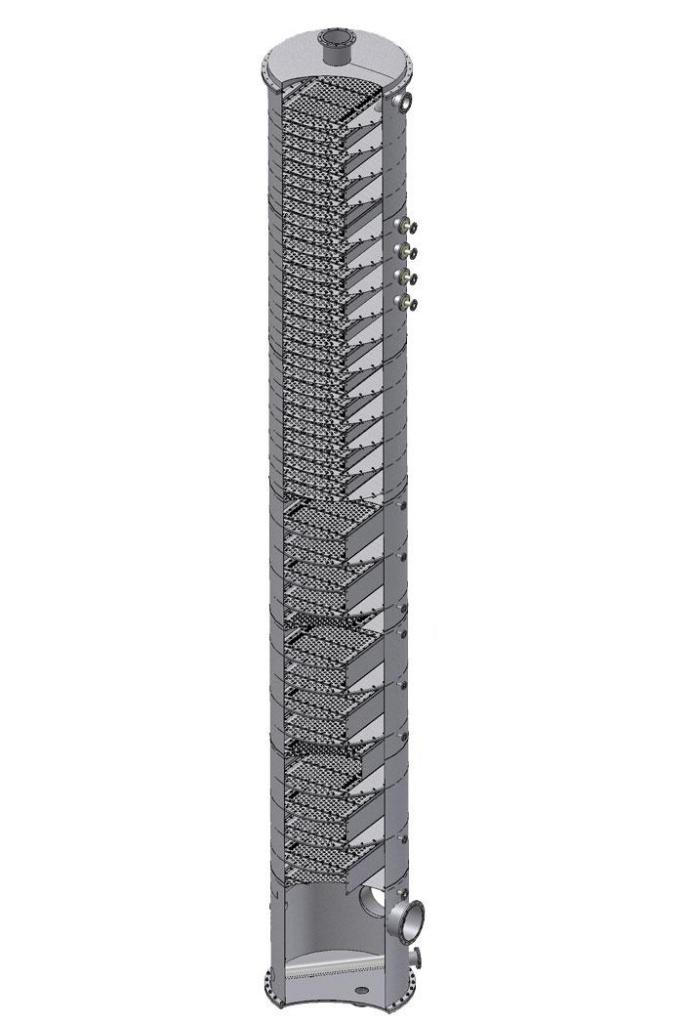
Its device differs from the usual "grandmother" moonshine in the presence of a vertical shaft, where alcohol-containing vapors enter before being in the refrigerator. In the mine, the vapor passes through a series of obstacles in the form of plates, loose parts of arbitrary shape, etc. The task of these obstacles is to cool the vapor a little and make it condense. In the form of droplets, it settles and by gravity it falls back into the tank precisely water - like a liquid with a higher boiling point. Alcohol vapors continue on their way to the top of the column and only there they are selected for cooling and condensation.
other methods
However, other methods are known for how to separate water and alcohol. For example, what will happen if the initial liquid is not heated, but rather cooled? According to some assumptions, this is exactly what the ancient Vikings did with their ale to make it stronger. In a jug with ale, which was carried out at night in severe frost, an ice formed by morning. They threw it away, and the drink remaining in the jug turned out to be stronger and more picky. The secret of the method is simple - water crystallizes at a temperature of 0 ° C to freeze ethyl alcohol, it would have to be cooled to minus 115 ° C.

But if at all to set aside the temperature? In this case, how to separate alcohol and water? Chemistry can also help in solving the problem. There are substances that chemically bind water, neutral to alcohol. Others, on the contrary, react with alcohol, ignoring H 2 O. These are methods such as leaching or salting out. However, in practice, such methods are most often used only for scientific purposes.