Helium is an inert gas of the 18th group of the periodic table. This is the second easiest element after hydrogen. Helium is a gas without color, odor and taste, which becomes liquid at a temperature of -268.9 ° C. The boiling and freezing points are lower than any other known substance. This is the only element that does not harden when cooled under normal atmospheric pressure. For helium to become solid, 25 atmospheres are needed at a temperature of 1 K.
Discovery story
Helium was found in the gaseous atmosphere surrounding the Sun by the French astronomer Pierre Jansen, who in 1868 during an eclipse discovered a bright yellow line in the spectrum of the solar chromosphere. It was originally assumed that this line represented the sodium element. In the same year, the English astronomer Joseph Norman Lockyer observed a yellow line in the solar spectrum, which did not correspond to the known sodium lines D 1 and D 2 , and therefore he called it the D 3 line. Locker concluded that it was caused by matter on the Sun, unknown on Earth. He and the chemist Edward Frankland used the Greek name for the sun, "Helios," in the name of the element.
In 1895, the British chemist Sir William Ramsay proved the existence of helium on Earth. He obtained a sample of the uraniferous mineral cleveite, and after studying the gases formed during its heating, he found that the bright yellow line in the spectrum coincides with the D 3 line observed in the spectrum of the Sun. Thus, the new element was finally installed. In 1903, Ramsay and Frederick Soddu determined that helium is a product of the spontaneous decay of radioactive substances.
Spread in nature
Helium mass is about 23% of the total mass of the Universe, and the element is the second most abundant in space. It is concentrated in stars, where it is formed from hydrogen as a result of thermonuclear fusion. Although helium is found in the Earth’s atmosphere at a concentration of 1 part per 200 thousand (5 ppm) and is found in small amounts in radioactive minerals, meteorite iron, and also in mineral springs, large amounts of the element are found in the United States (especially Texas, New Mexico, Kansas, Oklahoma, Arizona and Utah) as a component (up to 7.6%) of natural gas. Its small reserves were discovered in Australia, Algeria, Poland, Qatar and Russia. In the earth's crust, the concentration of helium is only about 8 parts per billion.
Isotopes
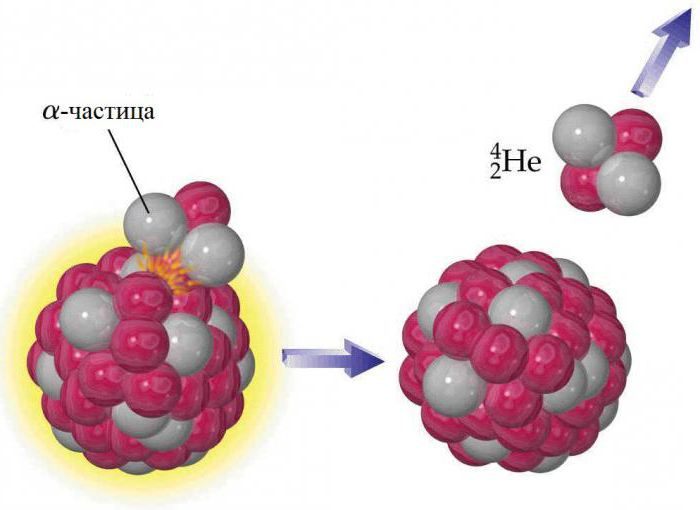
The nucleus of each helium atom contains two protons, but, like other elements, it has isotopes. They contain from one to six neutrons, so their mass numbers are in the range from three to eight. Stable of these are elements in which the helium mass is determined by the atomic numbers 3 ( 3 He) and 4 ( 4 He). All the rest are radioactive and very quickly decompose into other substances. Earth's helium is not the original component of the planet, it was formed as a result of radioactive decay. Alpha particles emitted by nuclei of heavy radioactive substances are nuclei of the 4 He isotope. Helium does not accumulate in large quantities in the atmosphere, because the Earth's gravity is not enough to prevent its gradual leakage into space. Traces of 3 He on Earth are explained by the negative beta decay of a rare element of hydrogen-3 (tritium). 4 He is the most common stable isotope: the ratio of 4 He to 3 He is about 700 thousand to 1 in the atmosphere and about 7 million to 1 in some helium-containing minerals.
Physical properties of helium
The boiling and melting points of this element are the lowest. For this reason, helium exists in the form of gas, with the exception of extreme conditions. He gaseous in water dissolves less than any other gas, and the diffusion rate through solids is three times greater than that of air. Its refractive index is closest to 1.
The thermal conductivity of helium is second only to the thermal conductivity of hydrogen, and its specific heat is unusually high. At ordinary temperatures, it expands during expansion, and cools below 40 K. Therefore, at T <40 K, helium can be converted into liquid by expansion.
An element is a dielectric if it is not in an ionized state. Like other noble gases, helium has metastable energy levels that allow it to remain ionized in an electric discharge when the voltage remains below the ionization potential.
Helium-4 is unique in that it has two liquid forms. The usual one is called helium I and exists at temperatures from the boiling point of 4.21 K (-268.9 ° C) to about 2.18 K (-271 ° C). Below 2.18 K, the thermal conductivity of 4 He becomes 1000 times greater than that of copper. This form is called helium II to distinguish it from the usual. It has superfluidity: viscosity is so low that it cannot be measured. Helium II spreads into a thin film on the surface of any substance it touches, and this film flows without friction even against gravity.
The less abundant helium-3 forms three different liquid phases, two of which are superfluids. Superfluidity in 4 He was discovered by the Soviet physicist Peter Leonidovich Kapitsa in the mid-1930s, and the same phenomenon in 3 He was first noticed by Douglas D. Osherov, David M. Lee, and Robert S. Richardson from the USA in 1972.
A liquid mixture of two helium-3 and -4 isotopes at temperatures below 0.8 K (-272.4 ° C) is divided into two layers - almost pure 3 He and a mixture of 4 He with 6% helium-3. The dissolution of 3 He in 4 He is accompanied by a cooling effect, which is used in the construction of cryostats, in which the temperature of helium drops below 0.01 K (-273.14 ° C) and is maintained for several days.
Connections
Under normal conditions, helium is chemically inert. In extreme conditions, it is possible to create compounds of an element that are not stable under normal conditions of temperature and pressure. For example, helium can form compounds with iodine, tungsten, fluorine, phosphorus, and sulfur when it is exposed to an electric glow discharge when bombarded by electrons or in a plasma state. Thus, HeNe, HgHe 10 , WHe 2 and the molecular ions He 2 + , He 2 ++ , HeH + and HeD + were created . This technique also made it possible to obtain neutral molecules of He 2 and HgHe.
Plasma
In the Universe, ionized helium is predominantly distributed, the properties of which differ significantly from the molecular one. Its electrons and protons are not connected, and it has a very high electrical conductivity even in a partially ionized state. The charged particles are strongly affected by magnetic and electric fields. For example, in a solar wind, helium ions, together with ionized hydrogen, interact with the Earth's magnetosphere, causing the northern lights.
Discovery of deposits in the USA
After drilling a well in 1903 in Dexter, Kansas, non-combustible gas was obtained. Initially, it was not known that it contained helium. What gas was found was determined by the geologist of the state of Erasmus Haworth, who collected its samples and at the University of Kansas with the help of chemists Cady Hamilton and David MacFarland found that it contains 72% nitrogen, 15% methane, 1% hydrogen and 12% was not identified. After conducting subsequent analyzes, scientists found that 1.84% of the sample is helium. So we learned that this chemical element is present in huge quantities in the bowels of the Great Plains, from where it can be extracted from natural gas.
Industrial production
This has made the United States a world leader in helium production. At the suggestion of Sir Richard Trelfall, the U.S. Navy funded three small pilot plants to produce this substance during World War I to provide balloons with lightweight non-combustible lifting gas. Under this program, a total of 5700 m 3 of 92% He was produced, although before that only less than 100 l of gas had been produced. Part of this volume was used in the world's first helium airship, the U.S. Navy S-7, which made its first flight from Hampton Roads (Virginia) to Bolling Field (Washington, DC) on December 7, 1921.
Although the process of low-temperature liquefaction of gas at that time was not sufficiently developed to be significant during the First World War, production continued. Helium was mainly used as lifting gas in aircraft. Demand for it grew during World War II, when it began to be used in shielded arc welding. The element was also important in the Manhattan atomic bomb project.
US National Stock
In 1925, the United States Government created the National Helium Reserve in Amarillo, Texas, to provide military airships during the war and commercial aircraft in peacetime. The use of gas after World War II was reduced, but the supply was increased in the 1950s to ensure, among other things, its supply as a coolant used in the production of oxygen-hydrogen rocket fuel during the space race and the Cold War. The use of helium in the United States in 1965 was eight times the peak consumption of wartime.
Following the adoption of the Helium Act of 1960, the Mining Bureau contracted 5 private enterprises to extract the element from natural gas. A 425 km gas pipeline was built for this program, connecting these plants with a government partially depleted gas field near Amarillo in Texas. The helium-nitrogen mixture was pumped into the underground storage and remained there until it became necessary.
By 1995, a billion cubic meter stock had been collected, and the National Reserve owed $ 1.4 billion, prompting the US Congress in 1996 to phase out it. After the adoption of the law on the privatization of helium in 1996, the Ministry of Natural Resources began liquidating the storage facility in 2005.
Cleanliness and production volumes
Helium, produced before 1945, had a purity of about 98%, the remaining 2% was nitrogen, which was sufficient for airships. In 1945, a small amount of 99.9 percent gas was produced for use in arc welding. By 1949, the purity of the obtained element reached 99.995%.
Over the years, the United States has produced more than 90% of global commercial helium. Since 2004, 140 million m 3 of it has been produced annually, 85% of which is in the USA, 10% was produced in Algeria, and the rest - in Russia and Poland. The main sources of helium in the world are the gas deposits of Texas, Oklahoma and Kansas.
Receipt process
Helium (98.2% purity) is isolated from natural gas by liquefying other components at low temperatures and at high pressures. Adsorption of other gases by chilled activated carbon makes it possible to achieve a purity of 99.995%. A small amount of helium is produced by large-scale liquefaction of air. About 900 tons of air can be obtained. m of gas.
Fields of application
Noble gas has found application in various fields.
- Helium, whose properties make it possible to obtain ultra-low temperatures, is used as a cooling agent in the Large Hadron Collider, superconducting magnets of MRI devices and nuclear magnetic resonance spectrometers, satellite equipment, as well as for liquefying oxygen and hydrogen in Apollo missiles.
- As an inert gas for welding aluminum and other metals, in the production of optical fiber and semiconductors.
- To create pressure in the fuel tanks of rocket engines, especially those that run on liquid hydrogen, since only gaseous helium retains its state of aggregation when hydrogen remains liquid);
- He-Ne gas lasers are used to scan barcodes at checkout counters in supermarkets.
- Helium-ion microscope allows you to get better images than electronic.
- Due to its high permeability, noble gas is used to check for leaks, for example, in automobile air conditioning systems, as well as to quickly fill airbags in a collision.
- Low density allows filling decorative balls with helium. Inert gas has replaced explosive hydrogen in airships and balloons. For example, in meteorology, helium balls are used to lift measuring instruments.
- In cryogenic technology it serves as a coolant, since the temperature of this chemical element in the liquid state is the lowest possible.
- Helium, whose properties provide it with low reactivity and solubility in water (and blood), in a mixture with oxygen, has found application in breathing compositions for scuba diving and coffer work.
- Meteorites and rocks are analyzed for the content of this element to determine their age.
Helium: Element Properties
The main physical properties of He are as follows:
- Atomic number: 2.
- Relative mass of helium atom: 4,0026.
- Melting point: no.
- Boiling point: -268.9 ° C.
- Density (1 atm, 0 ° C): 0.1785 g / p.
- Oxidation states: 0.