The simplest compounds studied in organic chemistry are saturated hydrocarbons or paraffins, also called alkanes. Their qualitative composition is represented by atoms of only two elements: carbon and hydrogen. Molecules of compounds contain only one type of chemical bond - single, or simple. In our article, we will study the structure, as well as methods for producing and properties of alkanes.
Representatives of the series and their names
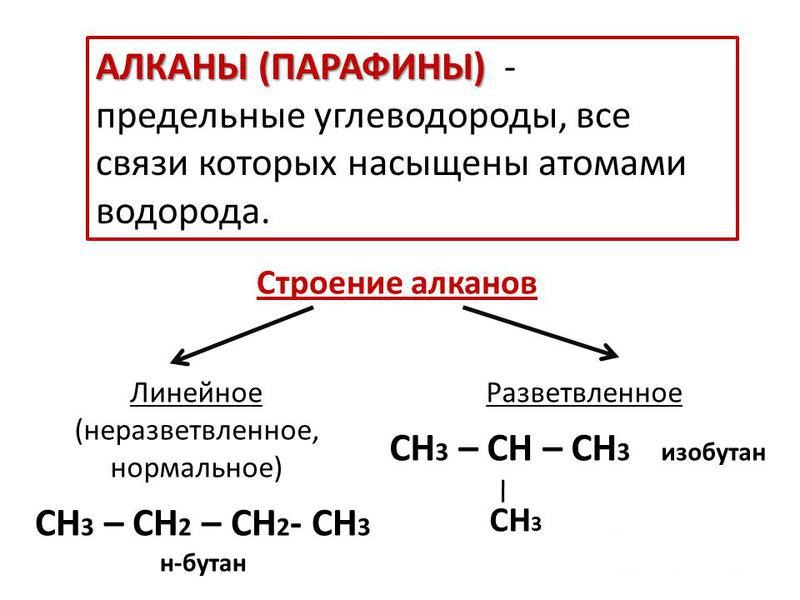
The first compound of the paraffin class is methane. Its molecular formula is CH 4 , it corresponds to the general formula of substances, which has the following form: C n H 2 n +2 . The first four alkanes have individual names, for example, methane, ethane. Starting from the fifth compound, the nomenclature is constructed using Greek numerals. For example, a substance with five carbon atoms in a C 5 H 12 molecule is called pentane (five from the Greek word "penta"). According to the rational nomenclature, alkanes, the chemical properties and the preparation of which we are studying, can be represented in the form of substances - methane derivatives. In its molecule, one or more hydrogen atoms are replaced by hydrocarbon radicals. According to the systematic nomenclature, one should choose the longest chain of carbon atoms, which are numbered from the end to which the radicals are closer. Then, the number of the carbon atom bound by the sigma bond to the radical particle is determined, and the radical is refined by adding to it the name of the alkane itself, for example, 3-methylbutane.
Getting alkanes
The main and most common source of paraffin mining is minerals: natural gas and oil. Traces of methane along with hydrogen and nitrogen can be found in the composition of the swamp gas. Solid alkanes containing a large number of carbon atoms in the molecule are present in ozokerite. This is mountain wax, which has a whole range of unique properties, the deposits of which are developed, for example, in Western Ukraine. There are also a number of synthetic methods for the production of saturated hydrocarbons, in particular, a reduction reaction. In industry, there are several methods for producing alkanes using redox reactions, for example, between halogenated alkyl and hydrogen iodide or sodium amalgam. More simple is the reduction of alkenes, alkynes or alkadienes with hydrogen in the presence of a nickel catalyst. The reaction product is the corresponding paraffin. The process can be expressed by the following reaction equation:
CH 2 = CH 2 + H 2 = H 3 C-CH 3 (ethane)
Alkaline melting of carboxylic acid salts
If heated sodium salt of CH 3 COONa or other substances of this class, which include active metal atoms, with sodium hydroxide or soda lime, you can get saturated hydrocarbons. The first type of reaction is often used in laboratory conditions, the second is used to accurately analyze the structure of carboxylic acid, which is part of the salt. This method of producing alkanes allows one to observe the splitting of the carbon chain of the reagent and the decrease in the number of carbon atoms in it.
Würz reaction
Substances that are derivatives of paraffins, in which hydrogen atoms are replaced by particles of chlorine, bromine or iodine, can interact with finely divided metallic sodium. The reaction equation in general form will be as follows:
2RHal + 2Na → R — R + 2NaHal,
This process was discovered in 1870 by the French chemist F. Wurz. Later P.P. Sharygin specified his mechanism leading to alkane production. It turned out that the halogen atom is first replaced by a metal. Then, the resulting organo-sodium substance interacts with another halogen alkane molecule. This reaction has found application in technology for the synthesis of higher paraffins.
Properties of saturated hydrocarbons
The physical characteristic of each class of organic compounds is determined by properties that change naturally, and depends on the structure of the molecules of substances. So, the first four homologs of alkanes, the reactions of which we examined earlier, are gases. Paraffins containing from 5 to 14 carbon atoms in their composition exist in the liquid phase, while the remaining alkanes are solid compounds. Gaseous and solid substances are odorless, liquid paraffins smell like kerosene or gasoline. The most important chemical properties of substances include, for example, severe oxidation - combustion, as a result of which a large amount of heat is released:
CH 4 + 2O 2 = CO 2 + 2H 2 O
Recall that methane is the main component of the main fuel - natural gas.
Substitution Reactions
Halogenation, which takes place according to the free radical mechanism, is another feature of alkanes. It relates to substitution reactions and leads to the formation of compounds - halogenated paraffins:
C 5 H 12 + Cl 2 = HCl + C 5 H 11 Cl (chloropentane).
Nitration is the interaction of alkanes with dilute nitric acid in the presence of a catalyst and under pressure, discovered in 1889 by N. M. Konovalov. Paraffin nitro compounds have a wide range of applications as raw materials for the production of rocket fuel, explosives, as well as for the production of carboxylic acids and amines.
Oxidation of the higher members of the homologous series of alkanes in the presence of a catalyst leads to the production of alcohols and carboxylic acids, which are used for the synthesis of plasticizers used in the production of plastics and detergents.
In our article, we examined the properties of saturated hydrocarbons and studied the methods for their preparation.