In everyday life, we all now and then encounter phenomena that accompany the processes of transition of substances from one state of aggregation to another. And most often we have to observe such phenomena on the example of one of the most common chemical compounds - well-known and familiar water for everyone. From the article you will learn how liquid water turns into solid ice - a process called water crystallization - and what features this transition is characterized by.
What is a phase transition?
Everyone knows that in nature there are three main aggregate states (phases) of a substance: solid, liquid, and gaseous. Often a fourth state is added to them - plasma (due to the features that distinguish it from gases). However, in the transition from gas to plasma there is no characteristic sharp boundary, and its properties are determined not so much by the relationship between the particles of matter (molecules and atoms), but by the state of the atoms themselves.
All substances, passing from one state to another, under ordinary conditions, abruptly, abruptly change their properties (the exception is some supercritical states, but here we will not concern them). Such a transformation is a phase transition, more precisely, one of its varieties. It occurs with a certain combination of physical parameters (temperature and pressure), called the phase transition point.
The transformation of a liquid into a gas is evaporation; the opposite is condensation. The transition of a substance from a solid to a liquid state is melting, but if the process goes in the opposite direction, then it is called crystallization. A solid body can immediately turn into gas and, conversely, in these cases they talk about sublimation and desublimation.
During crystallization, water turns into ice and clearly demonstrates how much its physical properties change. Let us dwell on some important details of this phenomenon.
The concept of crystallization
When the liquid solidifies upon cooling, the nature of the interaction and arrangement of the particles of the substance changes. The kinetic energy of the random thermal motion of its constituent particles decreases, and they begin to form stable bonds between themselves. When, thanks to these bonds, molecules (or atoms) line up in a regular, ordered manner, the crystalline structure of a solid forms.
Crystallization does not simultaneously cover the entire volume of the liquid being cooled, but begins with the formation of small crystals. These are the so-called crystallization centers. They grow in layers, stepwise, by joining all new molecules or atoms of matter along the growing layer.
Crystallization conditions
Crystallization requires cooling the liquid to a certain temperature (it is also a melting point). Thus, the crystallization temperature of water under normal conditions is 0 ° C.
For each substance, crystallization is characterized by the amount of latent heat. This is the amount of energy released during a given process (and in the opposite case, respectively, absorbed energy). The specific heat of crystallization of water is the latent heat released by one kilogram of water at 0 ° C. Of all the substances near water, it is one of the highest and amounts to about 330 kJ / kg. Such a large value is due to structural features that determine the parameters of water crystallization. The formula for calculating latent heat we will use below, after considering these features.
To compensate for latent heat, it is necessary to subcool the liquid so that crystal growth begins. The degree of supercooling has a significant effect on the number of crystallization centers and on the rate of their growth. While the process is ongoing, further cooling of the temperature of the substance does not change.
Water molecule
To better understand how water crystallizes, you need to know how the molecule of this chemical compound is structured, because the structure of the molecule determines the characteristics of the bonds it forms.
One oxygen atom and two hydrogen atoms are combined in a water molecule. They form an obtuse isosceles triangle in which an oxygen atom is located at the top of an obtuse angle of 104.45 °. In this case, oxygen strongly pulls the electron clouds in its direction, so that the molecule is an electric dipole. The charges in it are distributed over the vertices of an imaginary tetrahedral pyramid - a tetrahedron with internal angles of approximately 109 °. As a result, the molecule can form four hydrogen (proton) bonds, which, of course, affects the properties of water.
Features of the structure of liquid water and ice
The ability of a water molecule to form proton bonds is manifested both in liquid and in solid state. When water is a liquid, these bonds are rather unstable, easily destroyed, but also constantly form again. Due to their presence, water molecules are more closely related than particles of other liquids. Associating, they form special structures - clusters. For this reason, the phase points of the water are shifted toward higher temperatures, because energy also is needed to destroy such additional associates. Moreover, the energy is quite significant: if there were no hydrogen bonds and clusters, the crystallization temperature of water (as well as its melting) would be –100 ° C, and boiling point +80 ° C.
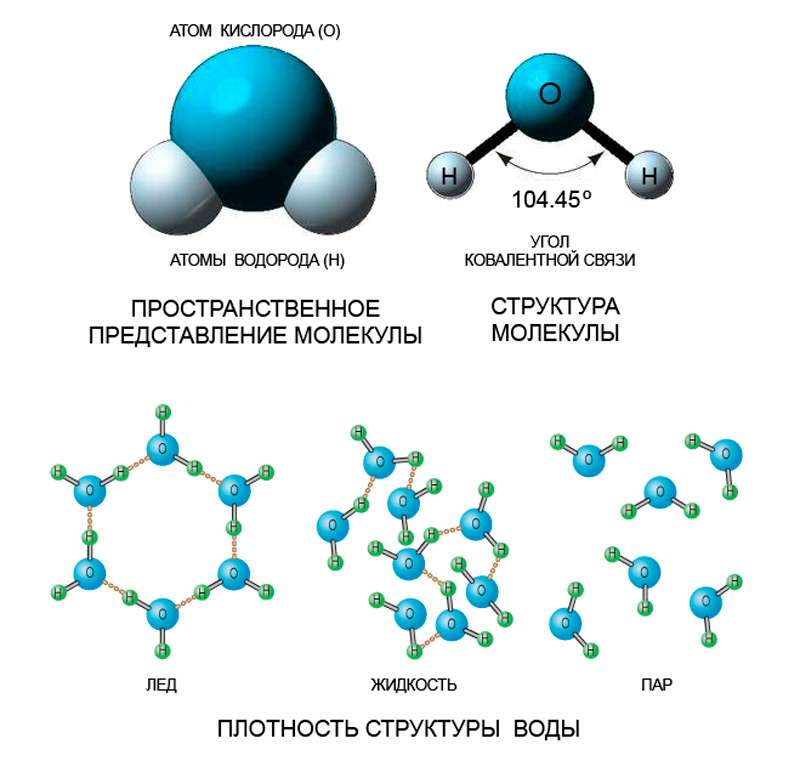
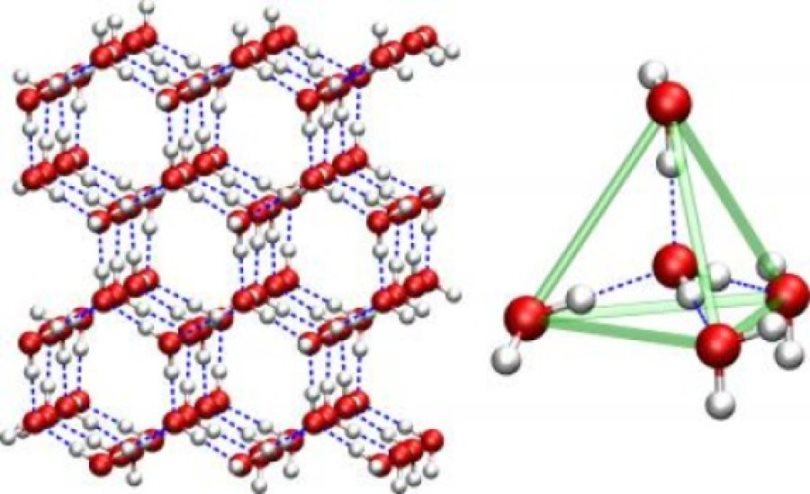
The structure of clusters is identical to the structure of crystalline ice. Binding each to four neighbors, water molecules build an openwork crystalline structure with a hexagonal base. Unlike liquid water, where microcrystals - clusters - are unstable and mobile due to the thermal movement of molecules, when ice is formed, they are rearranged in a stable and regular way. Hydrogen bonds fix the mutual arrangement of the nodes of the crystal lattice, and as a result, the distance between the molecules becomes somewhat larger than in the liquid phase. This circumstance explains the jump in the density of water during its crystallization - the density drops from almost 1 g / cm 3 to about 0.92 g / cm 3 .
About latent warmth
Features of the molecular structure of water are very seriously reflected in its properties. This can be seen, in particular, from the high specific heat of crystallization of water. It is due precisely to the presence of proton bonds, which distinguishes water from other compounds that form molecular crystals. It has been established that the hydrogen bond energy in water is about 20 kJ per mole, that is, 18 g. A significant part of these bonds is established "in bulk" when water freezes - this is where such a large energy return comes from.
We give a simple calculation. Let 1650 kJ of energy be released during crystallization of water. This is a lot: equivalent energy can be obtained, for example, by exploding six grenade lemon-f-1s. We calculate the mass of water subjected to crystallization. The formula relating the amount of latent heat Q, mass m and specific heat of crystallization λ is very simple: Q = - λ * m. The minus sign simply means that heat is given away by the physical system. Substituting the known values, we obtain: m = 1650/330 = 5 (kg). Only 5 liters is needed for as much as 1650 kJ of energy to be released during the crystallization of water! Of course, energy is not given out instantly - the process lasts for a rather long time, and the heat dissipates.
For example, many birds are well aware of this property of water, and use it to warm themselves near the freezing water of lakes and rivers, in such places the air temperature is several degrees higher.
Crystallization of solutions
Water is a wonderful solvent. The substances dissolved in it shift the crystallization point, as a rule, downward. The higher the concentration of the solution, the freezing will occur at a lower temperature. A striking example is seawater, in which many different salts are dissolved. Their concentration in the water of the oceans is 35 ppm, and such water crystallizes at –1.9 ° C. The salinity of water in different seas is very different, therefore, the freezing point is different. So, the Baltic water has a salinity of not more than 8 ppm, and its crystallization temperature is close to 0 ° C. Mineralized groundwater also freezes at temperatures below zero. It should be borne in mind that we are always talking only about the crystallization of water: sea ice is almost always fresh, in extreme cases slightly salted.

Aqueous solutions of various alcohols also have a lower freezing point, and their crystallization does not occur spasmodically, but with a certain temperature range. For example, 40 percent alcohol begins to freeze at -22.5 ° C, and finally crystallizes at -29.5 ° C.
But a solution of an alkali such as caustic soda NaOH or caustic is an interesting exception: it is characterized by an increased crystallization temperature.
How does clear water freeze?
In distilled water, the cluster structure is broken due to evaporation during distillation, and the number of hydrogen bonds between the molecules of such water is very small. In addition, in such water there are no impurities such as suspended microscopic dust particles, bubbles, etc., which represent additional centers of crystal formation. For this reason, the crystallization point of distilled water is lowered to –42 ° C.
Distilled water can be subcooled even up to –70 ° C. In such a state, supercooled water is able to crystallize almost instantly over the entire volume at the slightest shock or insignificant impurity.
Paradoxical hot water
An amazing fact - hot water goes into crystalline faster than cold water - was called the "Mpemba effect" in honor of a Tanzanian schoolboy who discovered this paradox. More precisely, they knew about him in antiquity, however, without finding an explanation, natural philosophers and natural scientists eventually ceased to pay attention to a mysterious phenomenon.
In 1963, Erasto Mpemba was surprised that the heated ice cream mixture hardens faster than cold. And in 1969, an intriguing phenomenon was confirmed already in a physical experiment (by the way, with the participation of Mpemba himself). The effect is explained by a whole range of reasons:
- more crystallization centers, such as air bubbles;
- high heat dissipation of hot water;
- high rate of evaporation, entailing a decrease in the volume of liquid.
Pressure as a crystallization factor
The relationship between pressure and temperature as key variables affecting the process of crystallization of water is clearly reflected in the phase diagram. It can be seen from it that with increasing pressure the temperature of the phase transition of water from liquid to solid decreases extremely slowly. Naturally, the opposite is also true: the lower the pressure, the higher the temperature is needed for the formation of ice, and it grows just as slowly. To achieve conditions under which water (not distilled!) Is able to crystallize in ordinary Ih ice at the lowest possible temperature of –22 ° C, the pressure must be increased to 2085 atmospheres.
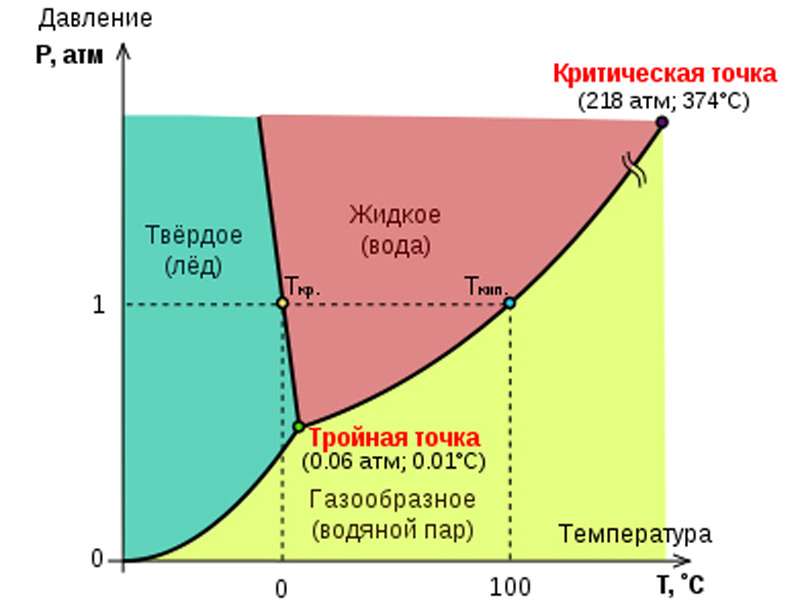
The maximum crystallization temperature corresponds to the following combination of conditions, called the triple point of water: 0.006 atmospheres and 0.01 ° C. With these parameters, the points of crystallization-melting and condensation-boiling coincide, and all three states of aggregation of water coexist in equilibrium (in the absence of other substances).
Many types of ice
Currently, about 20 modifications of the solid state of water are known - from amorphous to ice XVII. All of them, except ordinary Ih ice, require crystallization conditions that are exotic for the Earth, and far from all are stable. Only ice Ic is very rarely found in the upper layers of the earth's atmosphere, but its formation is not associated with freezing of water, since it is formed from water vapor at extremely low temperatures. Ice XI was found in Antarctica, but this modification is a derivative of ordinary ice.
By crystallizing water at extremely high pressures, it is possible to obtain such modifications of ice as III, V, VI, and, with a simultaneous increase in temperature, ice VII. It is likely that any of them can form under conditions unusual for our planet on other bodies of the solar system: on Uranus, Neptune or large satellites of giant planets. Presumably, future experiments and theoretical studies of the properties of these ices, which have been little studied, as well as features of the processes of their crystallization, will clarify this issue and will reveal much more.