Aldehydes is a word that seems scary to many. It is clearly associated with chemistry, possibly poisonous substances. Formaldehydes are a close term that causes particular fear in people. Is it possible to get cancer if exposed to the same substances? Is it possible to get poisoned? How does it affect life and health? Do aldehydes surround us? Let's try to understand the features of the use of aldehydes and how dangerous they are.
Beautiful and fragrant
This will surprise many, but one of the areas of application of aldehydes is perfumery. The term refers to such compounds in the molecule of which there is hydrogen, carbon. They are widely found in the world around us. For the first time in the perfume industry, they were able to apply them back in 1905. Using connections is not easy. If the concentration is low, aldehydes give the aroma a light, pleasant, aesthetic - fruity, floral. But it’s worth a little too much, and it will smell only rancid oil. The correct use of these substances allows you to make the aroma of perfume rich, deep. The use of aldehydes allowed the production of toilet water with unusual and expensive odors - it would be completely unprofitable to use natural components for this. The result of the synthetic product is in no way inferior to natural ingredients.
Aldehydes are produced industrially, are synthetic compounds. Between themselves, they are quite different in structure, which means physical parameters, including smell. The smaller the mass of the molecule, the aroma is unpleasant, the higher - the more people like it. Due to this feature, the use of aldehydes in perfumes is currently practiced quite widely.
Natural and artificial
Aldehydes are not only man-made compounds. Some varieties of such substances can be found in natural sources. For example, the production and use of aldehydes from orange peel has gained popularity. Another source is rose essential oil. True, this is the exception rather than the rule - the main percentage of varieties is made in laboratories synthetically. The task requires a minimum of time, and the process itself is relatively uncomplicated, so today there are practically no spirits that would not contain these components.
At one time, the production and use of aldehydes in perfumes was a real revolution in this area. Substances were one of the first synthetic, widely used in the manufacture of perfume compositions. On the one hand, their smell was similar to natural substances, on the other hand, obtaining is much simpler. Thanks to aldehydes, perfumes received persistent notes: citrus, fruits, flowers.
Appearances and passwords
Thanks to the study of their physical properties, the use of aldehydes has become widespread. The boom happened when the spirits of Chanel No. 5 saw the light. This was not the first composition in which such synthetic substances were present, but it was with her that they gained popularity.
But for the first time perfumes with industrially manufactured aromatic components were released in 1905. Manufacturer - Armingeat brand. The name of the iconic aromatic composition is Reve D'Or. Even earlier, in 1882, on the basis of the studied properties of aldehydes, the use of these substances was practiced in the development of Fougere Royal perfumes. They were used in a vanishingly small amount; nevertheless, the fact itself remains significant for the history of chemistry and perfumery.
The study of the properties, the use of aldehydes became fashionable when Chanel was launched. Their legendary perfume was the most successful experiment with synthetic substances. Aldehydes are allocated about one percent of the total volume of liquid - no one has achieved such a high concentration before this company. By the way, for some time it was believed that perfumes appeared as a result of an erroneous misunderstanding, an oversight made by a perfumer - so many aldehydes simply could not be used. However, even if this was a mistake, it turned out to be surprisingly successful - Chanel No. 5 is today one of the most sought-after fragrances in the world.
Past and present
Properties, production, use of aldehydes - these topics have been of interest to perfumers all over the world for the second century. The perfumes created with these substances have changed, and it is currently hard to imagine what the very first Chanel No. 5 was. Three decades after the development of the original composition, the company decided to re-release the product, but as eau de toilette. It contained significantly less synthetic product - such a change in composition was made intentionally. Nevertheless, the initial aroma due to aldehydes became extravagant, to some extent determining the brand’s path for many years. The originality of these spirits to this day has been preserved in the legends of the perfumery world.
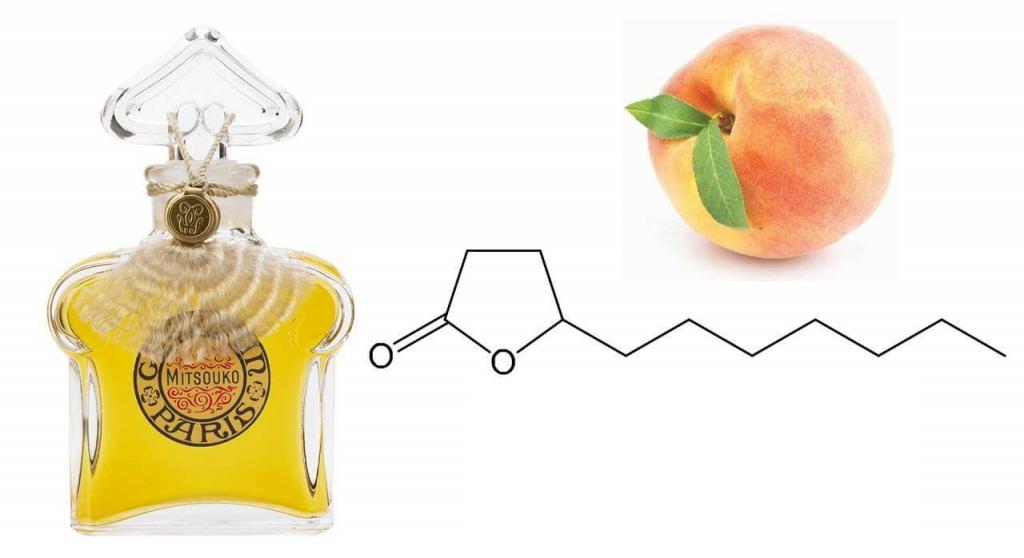
Perfumes as a field of application of aldehydes opened the world to their delicate and romantic aroma. Perfumes with the inclusion of these synthetic compounds began to sound fragile. Currently, there is even a separate category of aromas - floral-aldehyde. There are several varieties of these compounds that are used most often: hepta-, octa-, nona-, deca-, undecanal, undecalactone, lauryl aldehydes. Each of them has its own characteristics of smell. Some smell like green grass, others smell like oranges, rose, lemon. Undecanal is not only a synthetic substance - it was found in essential oils of different plants. Some aldehydes smell like violets, grapefruit, lilac, wax, peach. To create perfumes with notes of lily of the valley, you need to apply lilial, and the smell of daffodil will give the inclusion of phenylacetaldehyde. Perfumes as a field of application of aldehydes has become a platform for the development of complex combinations. This is how it was possible to obtain, for example, perfumes with a jasmine aroma - for this it is necessary to combine several compounds in different ratios.
Not only about the beautiful
Perfumes are far from the only field of application of aldehydes. Chemistry, medicine, industry - in all these areas different types of substances of the described group are needed. In particular, in industry, ethanal and methanal are most widely used. Metanal is necessary for the manufacture of phenol-formaldehyde resin - for this you will have to initiate a reaction involving phenol. Resin is the starting material for plastics. The use of aldehydes allows the inclusion in the reaction of specialized components, fillers - phenoplasts. If it is necessary to make a varnish, the above resin is mixed with alcohol, acetone until completely dissolved.
Urea resin, a necessary component for the production of aminos, can be obtained from methanal. A study of the structure, properties, preparation and use of aldehydes made it possible to reveal that amino plastics are the starting material for microporous raw materials, which have become indispensable in electrical engineering. But that's not all!
The use of aldehydes in medicine is quite diverse, and special attention should be paid to methanal. This compound is necessary for the manufacture of substances used in pharmaceuticals. In addition, dyes are obtained with its use.
Medicine and Chemistry
A study of the possibilities of using the chemical properties of aldehydes revealed that an aqueous solution consisting of 40% methanal can bring considerable benefits to a person. Modern scientists call it formalin. A distinctive feature is the coagulability of the protein under its influence. However, this quality has been applied not only for medical purposes. As experiments showed, if formalin is treated with skin, it gains firmness, and putrefactive processes are not afraid of it. This allowed the use of the substance in the leather industry.
In a similar way, the use of the most important methanaldehyde in medicine can be explained. Under the influence of formalin obtained from it, the protein coagulates, which means that it is possible to ensure a long shelf life of biological preparations. In some cases, formalin is the most suitable drug for the treatment, disinfection of seed material.
The second aldehyde widely used in industry is ethanal. Any chemist knows exactly where acetic aldehyde (ethanal) is used - it is indispensable for the manufacture of acetic acid, which is in demand for a huge variety of chemical processes.
Medicine: features of the use of aldehydes
Formalin (aka formaldehyde) is a colorless liquid that is easily recognizable by a specific odor. Studying the properties, structure, and use of aldehydes, scientists have found that formaldehyde is an excellent disinfectant. You can use it as a deodorizing. Formalin is used to clean hands, skin, if a person suffers from increased activity of sweat glands. For this, a one percent solution of the substance is used. A half-concentration is enough to sanitize tools. Formalin is included in the lysoform, used for douching in the ratio of 1: 2000 - 1: 3000.
A mixture of formalin, ethyl alcohol, cologne, purified water is formidron. Such a substance has been used as a local remedy when it is necessary to treat the skin of a person suffering from excessive sweating.
If you ask a medical student: “List the areas of application of aldehydes,” he will probably immediately remember formaldehyde ointment. It is a white substance with a slight odor of odorants and a characteristic formalin aroma. Such an ointment is used if sweat glands are too active. The drug is rubbed into the axillary fossa once a day. It can be used in the folds between the fingers, if this area also suffers from excessive sweating.
What else do you have?
Lysoform is prepared by mixing formaldehyde with alcohol and soap on potassium. Formalin and soap must be taken in equal proportions, and alcohol - half as much. The substance has a deodorizing effect, is a disinfectant. It is used when douching is necessary, therefore it is widespread in gynecology. Solutions with an active ingredient content of not more than 3% are used to disinfect hands.
Urotropin is a product derived from aldehyde. The substance is crystalline, has no smell, dissolves quickly in water, shows an alkaline reaction. Widely used in the treatment of infectious diseases of urine excretion pathways. In an acidic environment, the drug decomposes, one of the reaction products is formalin. Urotropin is used on an empty stomach. Individual reactions of intolerance, side effects of application are possible. For some (for example, irritation of the renal parenchyma), it is necessary to immediately stop the course of treatment. Urotropin is widely used for cholangitis, cholecystitis, skin and eye lesions of an allergic nature.
Urosal tablets have been created on aldehydes. In addition to urotropine, they contain phenyl salicylate.
Urotropin and calcium chloride are combined in the Calcex preparation. These are white tablets that dissolve quickly in water. They taste salty, bitter. The drug is indicated for colds. The optimal program of use is up to four times daily for a pair of tablets.
If necessary, locally suppress the activity of pathological microflora (gram-negative, gram-positive), you can use the drug "Cimal", also based on aldehydes. Its active components stimulate the healing of tissue damage, normalize the state of the epithelium. "Ciminal" is used externally, prescribed if the patient has pyoderma. "Ciminal" is suitable for the treatment of burns, non-healing ulcers, wounds. Available in two versions - suspension and powder. Active compounds are applied to diseased areas. The frequency of dressings is once every 3-4 days. Prolonged use can cause dermatitis. Local burning, itching are possible.
Chemical nuances
Despite such an extensive use of formalin in medicine, a predominant percentage of this substance is used for the manufacture of various plastics. An ordinary person rarely thinks about the use of aldehydes in everyday life, but without them, our life would be completely different: formaldehyde is indispensable for the manufacture of various parts of machines, electrical products. Acetaldehyde is a raw material for acetic acid, which is also widely used for a variety of processes and reactions. The reduction of this compound is a reaction resulting in ethyl alcohol. In some countries, this method of producing alcohol is currently quite common.
You can get aldehyde by oxidizing alcohol. One of the methods is the incandescence of a copper wire spiral in the fire of an alcohol lamp. Warming up, the subject acquires a dark coating - this is copper oxide. Once in a container with alcohol, the wire regains its luster. The process itself is accompanied by the appearance of a characteristic aldehyde smell. This method is a conditional description of the process of manufacturing aldehydes in an industrial environment. Special reactors are used in which copper and silver grids are installed. These elements heat and expel air saturated with methyl alcohol through them.
In the laboratory, aldehyde can be obtained using alcohol and various oxidizing agents. One option is potassium permanganate.
Acetaldehyde and its reaction products
The main field of application of acetic aldehyde (acetaldehyde) is the manufacture of acetic acid. This compound is actively used in everyday life, in the production of a variety of medications. Acetaldehyde is part of the popular Solcoderm drug, since it has been found that acetic acid burns warts, and is effective for condylomas. The possibility of its use against birthmarks, if the formation is benign, is proven.
You can buy a 30% solution of acetic acid - this concentration is more than enough for domestic purposes. If it is necessary to use a weaker version, you can use vinegar - in the composition of the acid product - 3-9%.
The scope of acetic aldehyde is the manufacture of glacial acid. This tool helps with warts, calluses, is used as a cauterizing. Glacial acid is also called pure. The main convenience of its use is the ability to independently dilute to the required concentration. Normally, the substance is crystalline, the melting point is 16.7 degrees Celsius, therefore, at normal room temperature, the compound is in the liquid phase. Glacial acid dissolves in alcohol, water, ether. This substance can dissolve phosphorus, tar, camphor. When protein interacts with it, coagulation occurs. In direct contact with the concentrated substance, chemical burns, blisters appear on the skin.
Varieties and forms
In diluted form, acetic acid is an essence. The content of the main component varies between 30-80%. The substance has established itself as a keratolytic, relieves itching, therefore, it is actively used in the manufacture of therapeutic ointments. Like formalin, the essence, one third consisting of acetic acid, helps with infection by fungi, foot mycosis. You can use the tool to disinfect insoles, shoes. The substance is applied to a cotton swab and wiped with it all the surfaces inside the shoe, then for a couple of hours they are placed in a tightly closed cellophane bag. Before use, shoes should be ventilated until the smell is gone.
A nine percent solution of a derivative of acetic aldehyde (acid) is table vinegar. It is used not only in cooking, but also when it is necessary to treat skin integuments. A few (not more than five) tablespoons of the liquid are taken into a glass of clean water, the resulting solution is used to treat the places bitten by insects, foci of urticaria, and itching.
Undiluted vinegar can be used if it is necessary to remove the lice nits that have multiplied in the hairline. Healers recommend using a vinegar to make a decoction of calamus roots. It is believed that this is effective in alopecia. Similarly, vinegar with nettle leaves is used.
Is it possible to get poisoned?
The product of acetic aldehyde - acetic acid - a substance with a surface effect, which differs from inorganic acids, but other features of the action are similar to this group.In case of poisoning, pairs of acids penetrate into the lungs, from where they are secreted, provoking severe pneumonia. With poisoning, hemolysis, hemoglobinuria are observed. The deadly volume for a person is considered to be 15 ml of anhydrous acid, 40 ml of essence, 300 ml of table vinegar.
At the autopsy of the deceased, acetic acid can be determined by a peculiar smell. Poisoning is accompanied by hepatic hemorrhage, necrotic foci, nephrosis with necrolysis, hemolysis.
Other types: features of use
The use of butyric aldehyde is primarily an organic synthesis reaction. The substance is indispensable in the manufacture of catalysts for the rubber vulcanization reaction. Butanal is also an aromatic additive, widely used in the food industry.
Isobutyric is an aldehyde form that is used as an intermediate. It is necessary in the synthesis of catalysts, rubber antioxidants. It is used in the manufacture of amino acids. Isobutyric aldehyde is in demand in perfumery, the manufacture of aromatic additives, plasticizers. It is used as an additive component of gasoline.
Crotonic - a form of aldehyde necessary for the manufacture of the same acid, surfactant. The substance is used in the production of chemotherapeutic compounds, pesticides. Crotonic aldehyde can dissolve polyvinyl chloride; therefore, it is used as a depressant in the vinyl polymerization reaction. Widespread connection in the manufacture of catalysts for rubber vulcanization. Crotonic aldehyde - a component of lubricants, a substance for tanning the skin. It is used as an indicator of some gases, it is used in the search for violation of the integrity of pipes, leaks.
Glutaraldehyde and ketals
Glutaraldehyde is a popular sterilizer. Its effectiveness against a wide range of microorganisms has been proven. It allows you to effectively get rid of spores, viruses. It can be used as a method of cold sterilization of medical devices and equipment. Chemical disinfector. In the leather industry - tannin. Glutaraldehyde is used as an element of fixatives, widely used for embalming. P-dioxane is a high-quality wood solvent used in dye baths and the textile industry. It is included in printing formulations, inks, as it is an effective wetting agent, dispersing agent. It was possible to apply it for the manufacture of cosmetic products, glue, paint removers, varnishes.
Ketals are compounds that are actively used as plasticizers, solvents. They are also used as intermediates in reactions that provoke the hardening of natural adhesives (for example, casein). Dichloroethyl formal is widely used as a solvent, an element of the reaction of manufacturing artificial rubber (polysulfide type). Dimethoxymethane is used to produce perfumes, fuels, and ointments. The substance dissolves coatings, glue.
How to minimize the danger?
An impressive percentage of aldehydes is volatile matter, for which there is an increased risk of fire. Even at room temperature, these liquids can form vapors, which under certain conditions can easily explode. To prevent explosion, fire, safety measures must be observed. This is especially important when working with the lowest of the aldehyde family. Compounds whose chain is either substituted or unsaturated are considered to be more dangerous.
When designing production facilities, it is important to think in advance how to minimize contact with aldehydes, how to prevent leakage. It is necessary to build fluid supply systems, as well as high-quality drainage, in order to minimize the consequences of leakage. If you plan to use aldehyde with carcinogenic qualities in production, you need to work with it in compliance with the rules of behavior with substances of this class.
An impressive percentage of known aldehydes can harm human eyes. During work, you need to use personal protection. For preventive maintenance use plastic face shields. If conditions allow, it is necessary to use aprons, special shoes, and hand protection. All production areas should be equipped with fountains so that workers can wash their eyes as needed. The company is responsible for organizing publicly accessible showers, staff training: employees should be able to use items to ensure personal protection.
Aldehydes and hazards
Most aldehydes known to people are dangerous. These substances can irritate the skin, respiratory organs, mucous membranes. To a greater extent, this is characteristic of the lower members of the family, with halogens in the substitution chain, unsaturated forms.
Some aldehydes have an analgesic effect, but it appears after dilution. The toxicity of aldehydes varies widely. For example, certain aldehydes from the aliphatic, aromatic groups are quickly separated, so there is no harm from them at all. Others are carcinogens, suspected carcinogens, which means that contact with them is very dangerous. There are chemical mutagens and capable of provoking a strong allergic reaction of the compound. Some aldehydes have sleeping pills.