Many modern technological devices and apparatuses were created due to the unique properties of substances found in nature. Mankind experimentally and carefully studying the elements surrounding us is constantly modernizing its own inventions - this process is called technical progress. It is based on elementary, accessible to everyone things that surround us in everyday life. For example, sand: what can be surprising and unusual in it? Scientists were able to isolate silicon from it - a chemical element without which computer technology would not exist. The scope of its application is diverse and is constantly expanding. This is achieved due to the unique properties of the silicon atom, its structure and the possibility of compounds with other simple substances.
Characteristic
In the periodic system developed by D.I. Mendeleev, silicon (a chemical element) is denoted by the symbol Si. Refers to non-metals, is located in the main fourth group of the third period, has atomic number 14. Its proximity to carbon is not accidental: in many respects their properties are comparable. It does not occur in nature in its pure form, as it is an active element and has fairly strong bonds with oxygen. The main substance is silica, which is an oxide, and silicates (sand). At the same time, silicon (its natural compounds) is one of the most common chemical elements on Earth. By the mass fraction of the content, it takes the second place after oxygen (more than 28%). The upper layer of the earth's crust contains silicon in the form of dioxide (this is quartz), various types of clays and sand. The second most common group is its silicates. At a depth of about 35 km from the surface, granite layers and basalt deposits are located, which include silicon compounds. The percentage in the earth’s core has not yet been calculated, but the mantle layers closest to the surface (up to 900 km) contain silicates. In the composition of sea water, the concentration of silicon is 3 mg / l, lunar soil 40% consists of its compounds. The open spaces that mankind has studied to date contain this chemical element in large quantities. For example, a spectral analysis of meteorites that were approaching the Earth at a distance accessible to researchers showed that they consist of 20% silicon. There is a likelihood of life forming on the basis of this element in our galaxy.

Research process
The history of the discovery of the chemical element of silicon has several stages. Many of the substances systematized by Mendeleev have been used by mankind for centuries. Moreover, the elements were in their natural form, i.e. in compounds that have not been chemically treated, and all their properties were not known to people. In the process of studying all the features of the substance, new directions of use appeared for him. The properties of silicon have not yet been fully studied - this element, with a wide enough and diverse range of applications, leaves room for new discoveries for future generations of scientists. Modern technologies will significantly accelerate this process. In the XIX century, many famous chemists tried to get silicon in its pure form. For the first time, L. Tenard and J. Gay-Lussac managed to do this in 1811, but the discovery of the element belongs to J. Berzelius, who was able not only to isolate the substance, but also to describe it. A chemist from Sweden received silicon in 1823, for this he used metallic potassium and potassium salt. The reaction occurred with the catalyst in the form of high temperature. The obtained simple substance of gray-brown color was amorphous silicon. The crystalline pure element was obtained in 1855 by St. Clair Deville. The complexity of the separation is directly related to the high strength of atomic bonds. In both cases, the chemical reaction is aimed at the process of purification from impurities, while the amorphous and crystalline models have different properties.

Silicon: chemical element pronunciation
The first name of the obtained powder - kiesel oil - was proposed by Berzelius. In the UK and the USA, silicon is still called nothing more than silicon (Silicium) or silicone (Silicon). The term comes from the Latin "flint" (or "stone"), and in most cases it is tied to the concept of "earth" due to its wide distribution in nature. The Russian pronunciation of this chemical is different, it all depends on the source. It was called silica (Zakharov used this term in 1810), Sicily (1824, Dvigubsky, Solovyov), silica (1825, Strakhov), and only in 1834 did the Russian chemist German Ivanovich Hess introduce the name that is still used today in most sources, silicon. In the periodic system of Mendeleev, it is indicated by the symbol Si. How is the chemical element silicon read? Many scientists in English-speaking countries pronounce its name as "si" or use the word "silicone". From here comes the world-famous name of the valley, which is a research and production site of computer equipment. The Russian-speaking population calls the element silicon (from the ancient Greek word "rock, mountain").
Being in the nature: deposits
Entire mountain systems are composed of silicon compounds, which in its pure form does not occur, because all known minerals are dioxides or silicates (aluminosilicates). Stones of amazing beauty are used by people as ornamental material - these are opals, amethysts, quartz of various types, jasper, chalcedony, agate, rock crystal, carnelian and many others. They were formed due to the inclusion of various substances in the composition of silicon, which determined their density, structure, color and direction of use. The entire inorganic world can be associated with this chemical element, which in the natural environment forms strong bonds with metals and non-metals (zinc, magnesium, calcium, manganese, titanium, etc.). Compared with other substances, silicon is quite readily available for mining on an industrial scale: it is found in most types of ore and minerals. Therefore, actively developed deposits are tied more to accessible energy sources than to territorial accumulations of matter. Quartzites and quartz sands are found in all countries of the world. The largest manufacturers and suppliers of silicon are: China, Norway, France, the USA (West Virginia, Ohio, Alabama, New York), Australia, South Africa, Canada, Brazil. All manufacturers use various methods that depend on the type of product (technical, semiconductor, high-frequency silicon). A chemical element, additionally enriched or, on the contrary, purified from all types of impurities, has individual properties on which its further use depends. This also applies to this substance. The structure of silicon determines the scope of its application.

Usage history
Very often, due to the similarity of names, people confuse silicon and flint, but these concepts are not identical. Let's make it clear. As already mentioned, pure silicon does not occur in nature, which cannot be said about its compounds (the same silica). The main minerals and rocks formed by the dioxide of the substance under consideration are sand (river and quartz), quartz and quartzite, feldspars and flint. Everyone must have heard of the latter, for he attaches great importance to the history of the development of mankind. The first tools created by people during the Stone Age are associated with this stone. Its sharp edges, formed when breaking away from the main breed, greatly facilitated the work of ancient housewives, and the possibility of sharpening - hunters and fishermen. Flint did not have the strength of metal products, but failed tools were easy to replace with new ones. Its use as a flint continued for many centuries - until the invention of alternative sources.
With regard to modern realities, the properties of silicon allow you to use the substance for decoration or creating ceramic dishes, while in addition to a beautiful aesthetic appearance, it has many excellent functional qualities. A separate area of its application is associated with the invention of glass about 3,000 years ago. This event made it possible to create mirrors, dishes, mosaic stained-glass windows from compounds containing silicon. The formula of the initial substance was supplemented with the necessary components, which made it possible to give the product the desired color and affect the strength of the glass. Amazing in beauty and diversity, works of art were made by man from minerals and stones containing silicon. The healing properties of this element were described by scientists of antiquity and have been used throughout the history of mankind. They laid out wells for drinking water, pantries for storing food, and used it both in everyday life and in medicine. The powder obtained by grinding was applied to the wounds. Particular attention was paid to water, which was infused in dishes made from compounds containing silicon. The chemical element interacted with its composition, which made it possible to destroy a number of pathogenic bacteria and microorganisms. And this is far from all industries where the substance under consideration is very, very much in demand. The structure of silicon determines its versatility.

The properties
For a more detailed acquaintance with the characteristics of the substance, it must be considered taking into account all possible properties. The characterization plan for the chemical element of silicon includes physical properties, electrophysical indicators, the study of compounds, reactions and conditions of their passage, etc. Silicon in crystalline form has a dark gray color with a metallic tint. The face-centered cubic lattice is similar to carbon (diamond), but due to the longer bond lengths it is not so strong. It is made plastic by heating to 800 ° C; in other cases, it remains fragile. The physical properties of silicon make this substance truly unique: it is transparent to infrared radiation. Melting point - 1410 0 , boiling point - 2600 0 , density under normal conditions - 2330 kg / m 3 . Thermal conductivity is unstable, for various samples it is taken at an approximate value of 25 0 C. The properties of the silicon atom allow it to be used as a semiconductor. This area of application is most in demand in the modern world. The value of electrical conductivity is affected by the composition of silicon and the elements that are in connection with it. So, for increased electronic conductivity antimony, arsenic, phosphorus are used, for perforated - aluminum, gallium, boron, indium. When creating devices with silicon, a surface treatment with a specific agent is used as a conductor, which affects the operation of the device.
The properties of silicon as an excellent conductor are used quite widely in modern instrumentation. Its application in the production of complex equipment (for example, modern computing devices, computers) is especially relevant.
Silicon: chemical characterization
In most cases, silicon is tetravalent; bonds are also found in which it can have a value of +2. Under normal conditions, it is inactive, has strong compounds, at room temperature it can only react with fluorine, which is in a gaseous state of aggregation. This is explained by the effect of blocking the surface with a dioxide film, which is observed when interacting with ambient oxygen or water. A catalyst must be used to stimulate reactions: an increase in temperature is ideal for a substance such as silicon. The chemical element interacts with oxygen at 400-500 0 , as a result, the dioxide film increases, the oxidation process is going on. When the temperature rises to 50 0 C, a reaction with bromine, chlorine, iodine is observed, as a result of which volatile tetrahalides are formed. Silicon does not interact with acids, with the exception of a mixture of hydrofluoric and nitric, and any alkali in a heated state is a solvent. Silicones are formed only by decomposition of silicides, it does not enter into a reaction with hydrogen. Compounds with boron and carbon are characterized by the highest strength and chemical passivity. A high resistance to alkalis and acids is associated with nitrogen, which occurs at temperatures above 1000 0 C. Silicides are obtained by reaction with metals, and in this case, the valency shown by silicon depends on an additional element. The formula of a substance formed with the participation of a transition metal is resistant to acids. The structure of a silicon atom directly affects its properties and ability to interact with other elements. The process of formation of bonds in nature and when exposed to a substance (in laboratory, industrial conditions) varies significantly. The structure of silicon suggests its chemical activity.
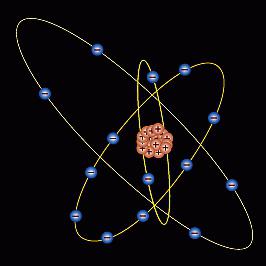
Structure
The structure of the silicon atom has its own characteristics. The core charge is +14, which corresponds to the serial number in the periodic system. The number of charged particles: protons - 14; electrons - 14; neutrons - 14. The structure diagram of a silicon atom has the following form: Si +14) 2) 8) 4. At the last (outer) level there are 4 electrons, which determines the degree of oxidation with a “+” or “-” sign. Silicon oxide has the formula SiO 2 (valency 4+), the volatile hydrogen compound is SiH 4 (valency -4). The large volume of the silicon atom allows in some compounds to have a coordination number of 6, for example, when combined with fluorine. The molar mass is 28, the radius of the atom is 132 pm, the configuration of the electron shell: 1S 2 2S 2 2P 6 3S 2 3P 2 .
Application
Surface or fully doped silicon is used as a semiconductor in the creation of many, including high-precision, devices (for example, solar photocells, transistors, current rectifiers, etc.). Ultrapure silicon is used to create solar panels (energy). The single-crystal form is used for the manufacture of mirrors and a gas laser. From compounds of silicon receive glass, ceramic tiles, dishes, porcelain, earthenware. The variety of the obtained types of goods is difficult to describe, their operation occurs at the household level, in art and science, in production. The resulting cement serves as raw material for the creation of building mixtures and bricks, finishing materials. The spread of oils, lubricants based on organosilicon compounds can significantly reduce the friction force in the moving parts of many mechanisms. Due to their unique properties in the field of counteracting aggressive environments (acids, temperatures), silicides are widely used in industry. Their electrical, nuclear and chemical indicators are taken into account by specialists in complex industries, and the structure of the silicon atom plays an important role.
We have listed the most high-tech and advanced applications today. The most common, manufactured in large volumes of technical silicon is used in a number of ways:
- As a raw material for the production of cleaner substances.
- For alloying alloys in the metallurgical industry: the presence of silicon increases refractoriness, increases corrosion resistance and mechanical strength (with an excess of this element, the alloy may be too brittle).
- As a deoxidant to remove excess oxygen from a metal.
- Raw materials for the production of silanes (silicon compounds with organic substances).
- For the production of hydrogen from an alloy of silicon with iron.
- Making solar panels.
Great importance of this substance and for the normal functioning of the human body. The structure of silicon, its properties are in this case decisive. At the same time, an excess or lack of it leads to serious diseases.
In the human body
Medicine has long been using silicon as a bactericidal and antiseptic. But with all the benefits of external use, this element should be constantly renewed in the human body. The normal level of its content will improve life activity in general. If it is deficient, more than 70 trace elements and vitamins will not be absorbed by the body, which will significantly reduce resistance to a number of diseases. The highest percentage of silicon is observed in bones, skin, and tendons. It plays the role of a structural element that maintains strength and gives elasticity. All skeletal hard tissues are formed due to its compounds. Recent studies have found silicon in the kidneys, pancreas, and connective tissues. The role of these organs in the functioning of the body is quite large, therefore, a decrease in its content will be detrimental to many basic indicators of life support. The body should receive 1 gram of silicon per day with food and water - this will help to avoid possible diseases, such as inflammatory processes of the skin, softening of bones, formation of stones in the liver, kidneys, impaired vision, condition of hair and nails, atherosclerosis. With a sufficient level of the content of this element, immunity increases, metabolic processes normalize, and the absorption of many elements necessary for human health improves. The greatest amount of silicon is in cereals, radishes, buckwheat. Silicon water will bring significant benefits. To determine the quantity and frequency of its use, it is better to consult a specialist.