Sulfur is a chemical element that is in the sixth group and third period of the periodic table. In this article, we will examine in detail its chemical and physical properties, production, use, and so on. The physical characteristic includes such signs as color, electrical conductivity, boiling point of sulfur, etc. The chemical describes its interaction with other substances.
Physics Sulfur
This is a fragile substance. Under normal conditions, it is in a solid state of aggregation. Sulfur has a lemon yellow color.
And for the most part, all of its compounds have yellow hues. It does not dissolve in water. It has low thermal and electrical conductivity. These signs characterize it as a typical non-metal. Despite the fact that the chemical composition of sulfur is not at all complicated, this substance can have several variations. It all depends on the structure of the crystal lattice, with which atoms are connected, but they do not form molecules.
So, the first option is rhombic sulfur. She is the most sustainable. The boiling point of sulfur of this type is four hundred forty-five degrees Celsius. But in order for this substance to go into a gaseous state of aggregation, it must first pass through a liquid. So, sulfur melts at a temperature of one hundred and thirteen degrees Celsius.
The second option is monoclinic sulfur. It is a needle-shaped crystals with a dark yellow color. The melting of sulfur of the first type, and then its slow cooling, leads to the formation of this species. This variety has almost the same physical characteristics. For example, the boiling point of sulfur of this type is the same four hundred forty-five degrees. In addition, there is such a variety of this substance as plastic. It is obtained by pouring rhombic heated almost to a boil in cold water. The boiling point of sulfur of this species is the same. But the substance has the ability to stretch like rubber.
Another component of the physical characteristic that I would like to talk about is the ignition temperature of sulfur.
This indicator may vary depending on the type of material and its origin. For example, the ignition temperature of technical sulfur is one hundred and ninety degrees. This is a pretty low rate. In other cases, the flash point of sulfur can be two hundred forty-eight degrees and even two hundred and fifty-six. It all depends on what material it was extracted from, what density it has. But we can conclude that the combustion temperature of sulfur is quite low, in comparison with other chemical elements, it is a flammable substance. In addition, sometimes sulfur can be combined into molecules consisting of eight, six, four or two atoms. Now, having examined sulfur from the point of view of physics, we move on to the next section.
Chemical characterization of sulfur
This element has a relatively low atomic mass, it is equal to thirty-two grams per mole. The characteristic of the sulfur element includes such a feature of this substance as the ability to have a different degree of oxidation. In this, it differs from, say, hydrogen or oxygen. Considering the question of what is the chemical characteristic of the sulfur element, it is impossible not to mention that, depending on the conditions, it exhibits both reducing and oxidizing properties. So, in order, we consider the interaction of a given substance with various chemical compounds.
Sulfur and simple substances
Simple are substances that have in their composition only one chemical element. Its atoms may combine into molecules, as, for example, in the case of oxygen, or may not combine, as is the case with metals. So, sulfur can react with metals, other non-metals and halogens.
Metal interaction
To carry out this kind of process, high temperature is required. Under such conditions, an addition reaction occurs. That is, metal atoms combine with sulfur atoms, forming complex sulfides. For example, if you heat two moles of potassium, mixing them with one mole of sulfur, you get one mole of sulfide of this metal. The equation can be written in the following form: 2K + S = K 2 S.
Reaction with oxygen
This is the burning of sulfur. As a result of this process, its oxide is formed. The latter can be of two types. Therefore, sulfur burning can occur in two stages. The first is when one mole of sulfur dioxide is formed from one mole of sulfur and one mole of oxygen. The equation of this chemical reaction can be written as follows: S + O 2 = SO 2 . The second stage is the addition of another oxygen atom to the dioxide. This happens if one mole of oxygen is added to two moles of sulfur dioxide under high temperature conditions. As a result, we obtain two moles of sulfur trioxide. The equation for this chemical interaction is as follows: 2SO 2 + O 2 = 2SO 3 . As a result of this reaction, sulfuric acid is formed. So, having carried out the two described processes, it is possible to pass the resulting trioxide through a stream of water vapor. And we get sulfate acid. The equation for such a reaction is written as follows: SO 3 + H 2 O = H 2 SO 4 .
Halogen interaction
The chemical properties of sulfur, like other non-metals, allow it to react with this group of substances. It includes compounds such as fluorine, bromine, chlorine, iodine. Sulfur reacts with any of them, with the exception of the latter. An example is the process of fluoridation of an element of the periodic table under consideration. By heating the aforementioned non-metal with halogen, two variations of fluoride can be obtained. The first case: if we take one mole of sulfur and three moles of fluorine, we get one mole of fluoride, the formula of which is SF 6 . The equation looks like this: S + 3F 2 = SF 6 . In addition, there is a second option: if we take one mole of sulfur and two moles of fluorine, we get one mole of fluoride with the chemical formula SF 4 . The equation is written as follows: S + 2F 2 = SF 4 . As you can see, it all depends on the proportions in which to mix the components. In exactly the same way, you can carry out the process of chlorination of sulfur (two different substances can also form) or bromination.

Interactions with other simple substances
The characteristic of the sulfur element does not end there. The substance may also undergo a chemical reaction with hydrogen, phosphorus and carbon. Due to the interaction with hydrogen, sulfide acid is formed. As a result of its reaction with metals, their sulfides can be obtained, which, in turn, are also obtained directly by the interaction of sulfur with the same metal. The addition of hydrogen atoms to sulfur atoms occurs only at very high temperatures. During the reaction of sulfur with phosphorus, its phosphide is formed. It has the following formula: P 2 S 3. In order to get one mole of a given substance, you need to take two moles of phosphorus and three moles of sulfur. During the interaction of sulfur with carbon, carbide of the considered nonmetal is formed. Its chemical formula is: CS 2 . In order to get one mole of this substance, you need to take one mole of carbon and two moles of sulfur. All the addition reactions described above occur only when the reactants are heated to high temperatures. We examined the interaction of sulfur with simple substances, now we will move on to the next point.
Sulfur and Complex Compounds
Complex substances are those substances whose molecules consist of two (or more) different elements. The chemical properties of sulfur allow it to react with compounds such as alkalis, as well as concentrated sulfate acid. Her reactions with these substances are quite peculiar. First, consider what happens when the considered non-metal is mixed with alkali. For example, if we take six moles of potassium hydroxide and add three moles of sulfur to them, we get two moles of potassium sulfide, one mole of sulfite of this metal and three moles of water. This kind of reaction can be expressed by the following equation: 6KOH + 3S = 2K 2 S + K2SO 3 + 3H 2 O. Interaction occurs by the same principle if sodium hydroxide is added . Next, we consider the behavior of sulfur when a concentrated solution of sulfate acid is added to it. If we take one mole of the first and two moles of the second substance, we get the following products: sulfur trioxide in an amount of three moles, and also water - two moles. This chemical reaction can only occur when the reagents are heated to a high temperature.
Getting the considered non-metal
There are several basic methods by which sulfur can be extracted from a variety of substances. The first method is to isolate it from pyrite. The chemical formula of the latter is FeS 2 . When this substance is heated to a high temperature without access to oxygen, another iron sulfide - FeS - and sulfur can be obtained. The reaction equation is written in the following form: FeS 2 = FeS + S. The second method for producing sulfur, which is often used in industry, is the burning of sulfur sulfide under the condition of a small amount of oxygen. In this case, it is possible to obtain the considered non-metal and water. For the reaction, you need to take the components in a molar ratio of two to one. As a result, we get the final products in the proportions of two to two. The equation of this chemical reaction can be written as follows: 2H 2 S + O 2 = 2S + 2H 2 O. In addition, sulfur can be obtained during various metallurgical processes, for example, in the production of metals such as nickel, copper and others.
Industrial use
The non-metal considered by us has found its widest application in the chemical industry. As mentioned above, here it is used to obtain sulfate acid from it. In addition, sulfur is used as a component for the manufacture of matches, due to the fact that it is a flammable material. It is also indispensable in the production of explosives, gunpowder, sparklers, etc. In addition, sulfur is used as one of the ingredients in pest control products. In medicine, it is used as a component in the manufacture of medicines for skin diseases. Also, the substance in question is used in the production of a variety of dyes. In addition, it is used in the manufacture of phosphors.
Electronic structure of sulfur
As you know, all atoms consist of a nucleus in which protons - positively charged particles - and neutrons, i.e. particles with a zero charge, are located. Electrons revolve around the nucleus, the charge of which is negative. For an atom to be neutral, its structure must have the same number of protons and electrons. If there are more of the latter, this is a negative ion - anion. If on the contrary - the number of protons is greater than the electrons - this is a positive ion, or cation. Sulfur anion may act as an acid residue. It is part of the molecules of substances such as sulfide acid (hydrogen sulfide) and metal sulfides. Anion is formed during electrolytic dissociation, which occurs when a substance is dissolved in water. In this case, the molecule decomposes into a cation, which can be represented as a metal ion or hydrogen, as well as a cation - an ion of an acid residue or a hydroxyl group (OH-).
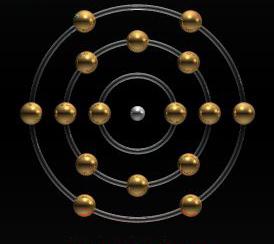
Since the serial number of sulfur in the periodic table is sixteen, we can conclude that it is precisely this number of protons that is in its core. Based on this, we can say that the electrons orbiting around are also sixteen. The number of neutrons can be found by subtracting the serial number of the chemical element from the molar mass: 32 - 16 = 16. Each electron does not rotate randomly, but in a specific orbit. Since sulfur is a chemical element that belongs to the third period of the periodic table, there are three orbits around the nucleus. The first of them has two electrons, the second has eight, the third has six. The electronic formula of the sulfur atom is written as follows: 1s2 2s2 2p6 3s2 3p4.
Prevalence in nature
Basically, the chemical element in question is found in minerals, which are sulfides of various metals. First of all, it is pyrite - a salt of iron; it is also lead, silver, copper luster, zinc blende, cinnabar - mercury sulfide. In addition, sulfur can also be a part of minerals, the structure of which is represented by three or more chemical elements.
For example, chalcopyrite, mirabilite, kizerite, gypsum. You can consider each of them in more detail. Pyrite is ferrum sulfide, or FeS
2 . It has a light yellow color with a golden sheen. This mineral can often be found as an admixture in lapis lazuli, which is widely used for the manufacture of jewelry. This is due to the fact that these two minerals often have a common deposit. Copper luster - chalcocyte, or chalcosine - is a bluish-gray substance, similar to a metal.
Lead luster (galena) and silver luster (argentite) have similar properties: they both resemble metals in appearance and have a gray color. Cinnabar is a brownish-red dull mineral with gray spots. Chalcopyrite, whose chemical formula is CuFeS
2 , is golden yellow; it is also called the golden blende. Zinc blende (sphalerite) can have a color from amber to fiery orange. Mirabilite - Na
2 SO
4 x10H
2 O - transparent or white crystals. It is also called
glauber salt, used in medicine. The chemical formula of kieserite is MgSO
4 xH
2 O. It looks like a white or colorless powder. The chemical formula of gypsum is CaSO
4 x2H
2 O. In addition, this chemical element is part of the cells of living organisms and is an important trace element.