The nineteenth century in the history of mankind is the century in which many sciences have been reformed, including chemistry. It was at this time that the periodic system of Mendeleev appeared, and with it the periodic law. It was he who became the basis of modern chemistry. The periodic system of D. I. Mendeleev is a systematization of elements that establishes the dependence of chemical and physical properties on the structure and charge of an atom of matter.
History
The beginning of Mendeleev’s periodic system was laid by the book “Correlation of Properties with the Atomic Weight of Elements” written in the third quarter of the 17th century. It reflected the basic concepts of known chemical elements (at that time there were only 63). In addition, in many of them the atomic masses were determined incorrectly. This greatly interfered with the discovery of D. I. Mendeleev.
Dmitry Ivanovich began his work by comparing the properties of elements. First of all, he took up chlorine and potassium, and only then he switched to working with alkali metals. Armed with special cards on which chemical elements were depicted, he repeatedly tried to assemble this “mosaic”: he laid it out on his desk in search of the necessary combinations and matches.
After much effort, Dmitry Ivanovich still found the regularity he was looking for and built the elements in periodic rows. Having received empty cells between the elements, the scientist realized that not all chemical elements were known to Russian researchers, and that he should give this world the knowledge in chemistry that had not yet been given by his predecessors.
Everyone knows the myth that Mendeleev’s periodic table appeared in a dream, and from memory he collected elements into a single system. This is, roughly speaking, a lie. The fact is that Dmitry Ivanovich worked on his work for a long time and with concentration, and this exhausted him greatly. While working on a system of elements, Mendeleev once fell asleep. Waking up, he realized that he had not finished the table, and rather continued to fill in the empty cells. His acquaintance, a certain Foreigner, a university teacher, decided that the periodic table had a dream and spread the rumor among his students. So this hypothesis appeared.
Fame
The periodic system of chemical elements of Mendeleev is a reflection of the periodic law created by Dmitry Ivanovich in the third quarter of the XIX century (1869). It was in 1869 at a meeting of the Russian chemical community that Mendeleev’s notification about the creation of a certain structure was read out. And in the same year the book "Fundamentals of Chemistry" was published, in which the periodic system of chemical elements of Mendeleev was first published. And in the book “The natural system of elements and its use to indicate the qualities of undiscovered elements” D. I. Mendeleev first mentioned the concept of “periodic law”.
The structure and rules for the placement of elements
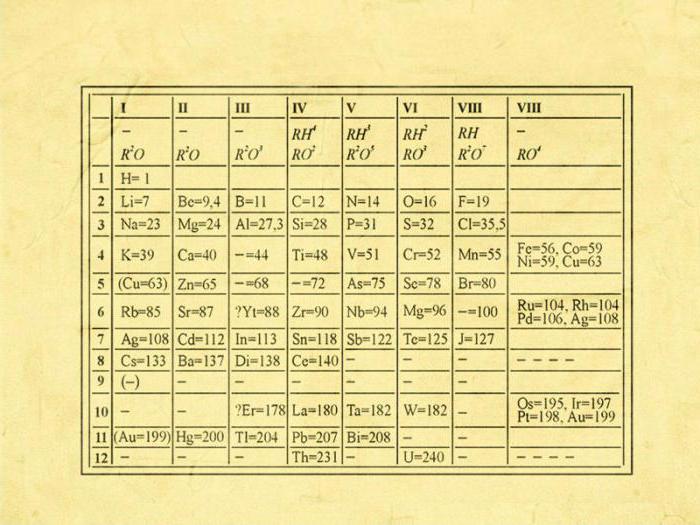
The first steps in creating the periodic law were made by Dmitry Ivanovich back in 1869-1871, while he worked hard to establish the dependence of the properties of these elements on the mass of their atom. The modern version is elements summarized in a two-dimensional table.
The position of the element in the table carries a certain chemical and physical meaning. By the location of the element in the table, you can find out what its valency is, determine the number of electrons and other chemical features. Dmitry Ivanovich tried to establish a connection between the elements, both similar in properties and different.
He based valency and atomic mass on the classification of chemical elements known at that time. Comparing the relative properties of the elements, Mendeleev tried to find a pattern that would combine all known chemical elements into one system. Having arranged them, based on an increase in atomic masses, he nevertheless achieved periodicity in each of the series.
Further development of the system
The periodic table that appeared in 1969 has been refined more than once. With the advent of noble gases in the 1930s, it turned out to reveal the latest dependence of the elements - not on the mass, but on the serial number. Later, it was possible to establish the number of protons in atomic nuclei, and it turned out that it coincides with the ordinal number of the element. Scientists of the 20th century studied the electronic structure of the atom. It turned out that it also affects the frequency. This greatly changed the idea of the properties of elements. This item was reflected in later editions of the periodic table of Mendeleev. Each new discovery of the properties and characteristics of elements organically fit into the table.
Characteristics of the periodic system of Mendeleev
The periodic table is divided into periods (7 rows arranged horizontally), which, in turn, are divided into large and small. The period begins with an alkali metal, and ends with an element with non-metallic properties.
Vertically, the table of Dmitry Ivanovich is divided into groups (8 columns). Each of them in the periodic system consists of two subgroups, namely, the main and secondary. After much debate on the proposal of D. I. Mendeleev and his colleague W. Ramzai, it was decided to introduce the so-called zero group. It contains inert gases (neon, helium, argon, radon, xenon, krypton). In 1911, scientists F. Soddy was invited to place in the periodic system and indistinguishable elements, the so-called isotopes - separate cells were allocated for them.

Despite the fidelity and accuracy of the periodic system, the scientific community did not want to acknowledge this discovery for a long time. Many great scientists ridiculed the activities of D.I. Mendeleev and believed that it was impossible to predict the properties of an element that had not yet been discovered. But after the alleged chemical elements were discovered (and these were, for example, scandium, gallium and germanium), the Mendeleev system and its periodic law became the theoretical basis of the science of chemistry.
Table in modern times
The periodic system of Mendeleev’s elements is the basis of most chemical and physical discoveries related to atomic-molecular theory. The modern concept of the element has developed precisely thanks to the great scientist. The appearance of the periodic system of Mendeleev introduced dramatic changes in the concept of various compounds and simple substances. The creation of a periodic system by scientists had a tremendous impact on the development of chemistry and all the sciences related to it.