Solid substances are those that can form bodies and have volume. They differ from liquids and gases in their shape. Solids retain their body shape due to the fact that their particles are not able to move freely. They differ in their density, ductility, electrical conductivity and color. They also have other properties. For example, most of these substances melt during heating, acquiring a liquid state of aggregation. Some of them, when heated, immediately turn into gas (sublimate). But there are also those that decompose into other substances.
Types of solids
All solids are divided into two groups.
- Amorphous, in which individual particles are arranged randomly. In other words: they do not have a clear (defined) structure. These solids are capable of melting in some set temperature range. The most common of these include glass and resin.
- Crystalline, which, in turn, are divided into 4 types: atomic, molecular, ionic, metal. In them, particles are located only according to a certain pattern, namely, in the nodes of the crystal lattice. Its geometry in different substances can vary greatly.
Solid crystalline substances prevail over amorphous in their numbers.
Types of crystalline solids
In the solid state, almost all substances have a crystalline structure. They differ in their structure. Crystal lattices in their nodes contain various particles and chemical elements. It was in accordance with them that they got their names. Each type has its characteristic properties:
- In an atomic crystal lattice, particles of a solid are linked by a covalent bond. It is distinguished by its strength. Due to this, such substances are characterized by a high melting point and boiling point. This type includes quartz and diamond.
- In a molecular crystal lattice, the bond between particles is distinguished by its weakness. Substances of this type are characterized by ease of boiling and melting. They are volatile, due to which they have a certain smell. Such solids include ice, sugar. The movements of molecules in solids of this type are distinguished by their activity.
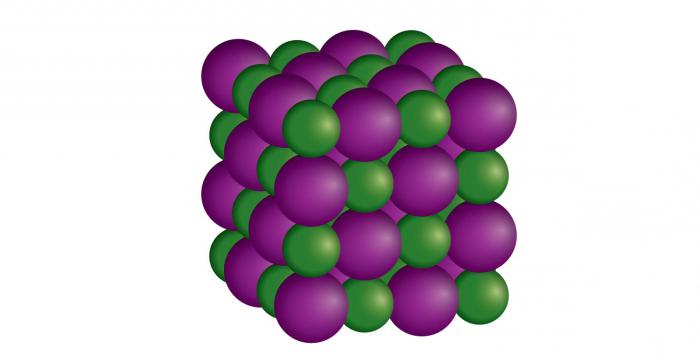
- In the ionic crystal lattice in the nodes, the corresponding particles are charged, positively and negatively charged. They are held by electrostatic attraction. This type of lattice exists in alkalis, salts, basic oxides. Many substances of this type are easily soluble in water. Thanks to a fairly strong bond between the ions, they are refractory. Almost all of them are odorless, since they are characterized by non-volatility. Substances with an ionic lattice are unable to conduct electric current, since they do not contain free electrons. A typical example of an ionic solid is salt. Such a crystal lattice gives it brittleness. This is due to the fact that any shift of it can lead to the appearance of ion repulsive forces.
- In the metal crystal lattice in the nodes, only ions of chemical substances are positively charged. Between them there are free electrons through which thermal and electrical energy perfectly pass. That is why any metals are distinguished by such a feature as conductivity.

General concepts of solids
Solids and substances are almost the same thing. These terms refer to one of the 4 aggregate states. Solids have a stable shape and the nature of the thermal motion of atoms. Moreover, the latter make small fluctuations near the equilibrium positions. The branch of science studying the composition and internal structure is called solid state physics. There are other important areas of expertise dealing with such substances. Change in shape under external influences and movement is called the mechanics of a deformable body.
Due to the various properties of solids, they have found application in various technical devices created by man. Most often, their use was based on such properties as hardness, volume, mass, elasticity, ductility, and brittleness. Modern science allows the use of other qualities of solids, which can be found exclusively in laboratory conditions.
What are crystals
Crystals are solids with particles arranged in a specific order. Each chemical substance has its own structure. Its atoms form a three-dimensionally periodic stack, called a crystal lattice. Solids have different structural symmetries. The crystalline state of a solid is considered stable, since it has a minimum amount of potential energy.
The vast majority of solid materials (natural) consists of a huge number of randomly oriented individual grains (crystallites). Such substances are called polycrystalline. These include technical alloys and metals, as well as many rocks. Monocrystalline is called single natural or synthetic crystals.
Most often, such solids are formed from the state of the liquid phase, represented by a melt or solution. Sometimes they are obtained from a gaseous state. This process is called crystallization. Thanks to scientific and technological progress, the procedure for growing (synthesis) of various substances has gained industrial scale. Most crystals have a natural shape in the form of regular polyhedrons. Their sizes are very different. So, natural quartz (rock crystal) can weigh up to hundreds of kilograms, and diamonds - up to several grams.
In amorphous solids, atoms are in constant vibration around randomly located points. They preserve a certain short-range order, but there is no long-range order. This is due to the fact that their molecules are located at a distance that can be compared with their size. The most common example of such a solid in our lives is the glassy state. Amorphous substances are often regarded as a liquid with infinitely high viscosity. The time of their crystallization is sometimes so long that it does not appear at all.
It is the above properties of these substances that make them unique. Amorphous solids are considered to be unstable, since they can become crystalline over time.
The molecules and atoms that make up a solid are packed with high density. They practically maintain their relative position with respect to other particles and stick together due to intermolecular interaction. The distance between the molecules of a solid in different directions is called the lattice parameter. The structure of a substance and its symmetry determine many properties, such as the electron zone, cleavage, and optics. When a solid substance is exposed to a sufficiently large force, these qualities can be violated to one degree or another. In this case, the solid is susceptible to permanent deformation.
Atoms of solids make oscillatory motions, which determine their possession of thermal energy. Since they are negligible, they can only be observed under laboratory conditions. The molecular structure of a solid largely affects its properties.
Solids
Features, properties of these substances, their qualities and particle motion are studied by various subdivisions of solid state physics.
For research are used: radio spectroscopy, structural analysis using x-rays and other methods. Thus, the mechanical, physical, and thermal properties of solids are studied. Hardness, resistance to loads, tensile strength, phase transformations are studied by material science. It largely echoes the physics of solids. There is another important modern science. The study of existing and the synthesis of new substances is carried out by solid state chemistry.
Features of solids
The nature of the motion of the external electrons of the atoms of a solid determines many of its properties, for example, electrical. There are 5 classes of such bodies. They are installed depending on the type of atomic bonds:
- Ionic, the main characteristic of which is the force of electrostatic attraction. Its features: reflection and absorption of light in the infrared region. At low temperatures, the ionic bond is characterized by low electrical conductivity. An example of such a substance is sodium hydrochloric acid (NaCl).
- Covalent, carried out by an electron pair that belongs to both atoms. Such a connection is divided into: single (simple), double and triple. These names indicate the presence of electron pairs (1, 2, 3). Double and triple bonds are called multiple. There is another division of this group. So, depending on the distribution of electron density, a polar and non-polar bond is isolated. The first is formed by different atoms, and the second - by the same. Such a solid state of matter, examples of which are diamond (C) and silicon (Si), is distinguished by its density. The hardest crystals relate specifically to covalent bonds.
- Metallic, formed by combining valence electrons of atoms. As a result, a common electron cloud arises, which shifts under the influence of electric voltage. A metal bond is formed when the bonded atoms are large. They are able to give electrons. In many metals and complex compounds this bond forms a solid state of the substance. Examples: sodium, barium, aluminum, copper, gold. Of the non-metallic compounds, the following can be noted: AlCr 2 , Ca 2 Cu, Cu 5 Zn 8 . Substances with a metal bond (metals) are diverse in their physical properties. They can be liquid (Hg), soft (Na, K), very hard (W, Nb).
- Molecular, arising in crystals that are formed by individual molecules of a substance. It is characterized by gaps between molecules with zero electron density. The forces that bind atoms in such crystals are significant. In this case, the molecules are attracted to each other only by weak intermolecular attraction. That is why the bonds between them are easily destroyed by heating. Compounds between atoms break down much more complicated. The molecular bond is subdivided into orientational, dispersive, and induction. An example of such a substance is solid methane.
- Hydrogen, which occurs between the positively polarized atoms of a molecule or its part and the negatively polarized smallest particle of another molecule or other part. These links include ice.
Solids Properties
What do we know today? Scientists have long been studying the properties of a solid state of matter. When exposed to temperatures, it also changes. The transition of such a body into a liquid is called melting. The transformation of a solid into a gaseous state is called sublimation. With decreasing temperature, crystallization of the solid occurs. Some substances under the influence of cold pass into the amorphous phase. Scientists call this process glass transition.
During phase transitions , the internal structure of solids changes. It acquires the highest ordering at lower temperatures. At atmospheric pressure and temperature T> 0 K, any substances that exist in nature harden. Only helium, whose crystallization requires a pressure of 24 atm, is an exception to this rule.
The solid state of a substance gives it various physical properties. They characterize the specific behavior of bodies under the influence of certain fields and forces. These properties are divided into groups. There are 3 methods of exposure, corresponding to 3 types of energy (mechanical, thermal, electromagnetic). Accordingly, there are 3 groups of physical properties of solids:
- Mechanical properties associated with stress and deformation of bodies. According to these criteria, solids are divided into elastic, rheological, strength and technological. At rest, such a body retains its shape, but it can change under the influence of an external force. Moreover, its deformation can be plastic (the initial form does not return), elastic (returns to its original form) or destructive (when a certain threshold is reached, decay / fracture occurs). Feedback on the applied force is described by elastic moduli. A solid body resists not only compression, stretching, but also shear, torsion, and bending. The strength of a solid is called its ability to resist destruction.
- Thermal, manifested when exposed to thermal fields. One of the most important properties is the melting point at which the body goes into a liquid state. It is noted in crystalline solids. Amorphous bodies have latent heat of fusion, since their transition to a liquid state with increasing temperature occurs gradually. Upon reaching a certain heat, an amorphous body loses its elasticity and acquires plasticity. This condition means that the glass transition temperature has been reached. When heated, deformation of the solid occurs. Moreover, it most often expands. Quantitatively, this condition is characterized by a certain coefficient. Body temperature affects mechanical characteristics such as fluidity, ductility, hardness and strength.
- Electromagnetic, associated with the impact on a solid of microparticle flows and electromagnetic waves of high rigidity. They conditionally include radiation properties.

Zone structure
Solids are also classified according to the so-called zone structure. So, among them there are:
- Conductors, characterized in that their conduction and valency zones overlap. In this case, electrons can move between them, receiving the slightest energy. Conductors include all metals. When a potential difference is applied to such a body, an electric current is generated (due to the free movement of electrons between the points with the smallest and greatest potential).
- Dielectrics whose zones do not overlap. The interval between them exceeds 4 eV. To carry electrons from the valence to the conduction band, a lot of energy is needed. Due to these properties, dielectrics practically do not conduct current.
- Semiconductors characterized by the absence of conduction and valence bands. The interval between them is less than 4 eV. To transfer electrons from the valence to the conduction band, less energy is needed than for dielectrics. Pure (undoped and intrinsic) semiconductors do not pass current well.
The movements of molecules in solids determine their electromagnetic properties.
Other properties
Solids are subdivided according to their magnetic properties. There are three groups:
- Diamagnetics, whose properties are little dependent on temperature or state of aggregation.
- Paramagnets resulting from the orientation of conduction electrons and magnetic moments of atoms. According to Curie's law, their susceptibility decreases in proportion to temperature. So, at 300 K it is 10 -5 .
- Bodies with an ordered magnetic structure, possessing the long-range order of atoms. At the nodes of their lattice, particles with magnetic moments are periodically located. Such solids and substances are often used in various fields of human activity.
The hardest substances in nature
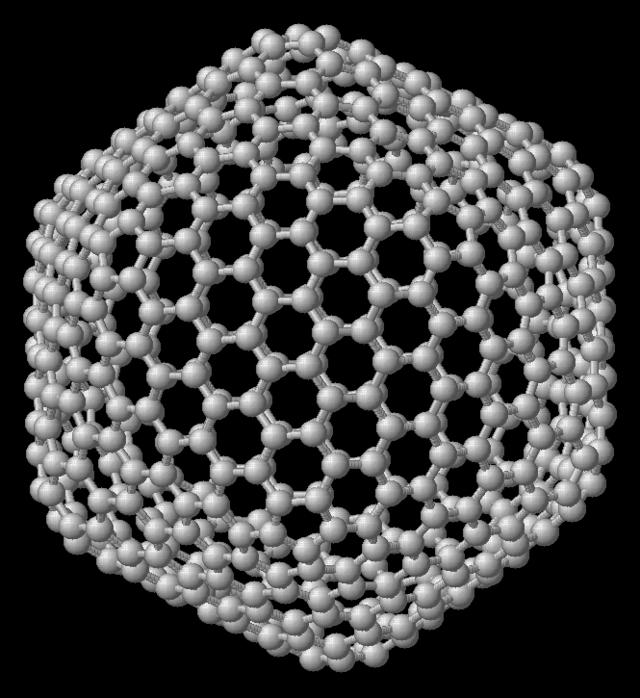
What are they like? The density of solids largely determines their hardness. In recent years, scientists have discovered several materials that claim to be the "most durable body." The hardest substance is fullerite (a crystal with fullerene molecules), which is about 1.5 times harder than diamond. Unfortunately, it is still available only in extremely small quantities.
To date, the hardest substance, which in the future may be used in industry, is lonsdaleite (hexagonal diamond). It is 58% harder than a diamond. Lonsdaleit is an allotropic modification of carbon. Its crystal lattice is very similar to diamond. The lonsdaleite cell contains 4 atoms and the diamond contains 8. Of the commonly used crystals, diamond remains the hardest to date.