The spatial structure of the molecules of inorganic and organic substances is of great importance in the description of their chemical and physical properties. If we consider a substance as a set of letters and numbers on paper, it is not always possible to come to the right conclusions. To describe many phenomena, especially those related to organic chemistry, it is necessary to know the stereometric structure of the molecule.
What is stereometry?
Stereometry is a section of chemistry that explains the properties of molecules of a substance based on its structure. Moreover, the spatial representation of molecules plays an important role here, because it is the key to unraveling many bioorganic phenomena.
Stereometry is a set of basic rules by which almost any molecule can be represented in bulk. The disadvantage of the gross formula written on a regular piece of paper is its inability to disclose a complete list of the properties of the substance under study.
An example is fumaric acid, which belongs to the class of dibasic. It is poorly soluble in water, not toxic and can be found in nature. However, if you change the spatial arrangement of COOH groups, you can get a completely different substance - maleic acid. It is well soluble in water, can only be obtained artificially, is a danger to humans due to toxic properties.
Vant-Hoff Stereochemical Theory
In the 19th century, M. Butlerov’s ideas about the flat structure of any molecule could not explain many properties of substances, especially organics. This served as an impetus to the writing by Vant-Hoff of the work “Chemistry in Space”, in which he supplemented M. Butlerov’s theory with his research in this field. He introduced the concept of the spatial structure of molecules, and also explained the importance of his discovery for chemical science.
So it was proved the existence of three types of lactic acid: meat-lactic, dextrorotatory and lactic acid fermentation. On a sheet of paper for each of these substances the structural formula will be the same, but the spatial structure of the molecules explains this phenomenon.
A consequence of the stereochemical theory of Vant-Hoff was the proof that the carbon atom is not flat, because its four valence bonds face the vertices of an imaginary tetrahedron.
Pyramidal spatial structure of organic molecules
Based on their findings by Vant-Hoff and his research, each carbon in the skeleton of organic matter can be represented as a tetrahedron. So we can consider 4 possible cases of the formation of CC bonds and explain the structure of such molecules.
The first case is when the molecule is a single carbon atom, which forms 4 bonds with hydrogen protons. The spatial structure of methane molecules almost completely repeats the outlines of the tetrahedron, but the valence angle is slightly changed due to the interaction of hydrogen atoms.
The formation of one chemical CC bond can be represented in the form of two pyramids, which are interconnected by a common peak. From such a construction of the molecule it can be seen that these tetrahedra can rotate around their axis and freely change position. If we consider this system as an example of an ethane molecule, the carbons in the skeleton are really able to rotate. However, from two characteristic positions, preference is given to energetically favorable when the hydrogens in the Newman projection do not overlap.
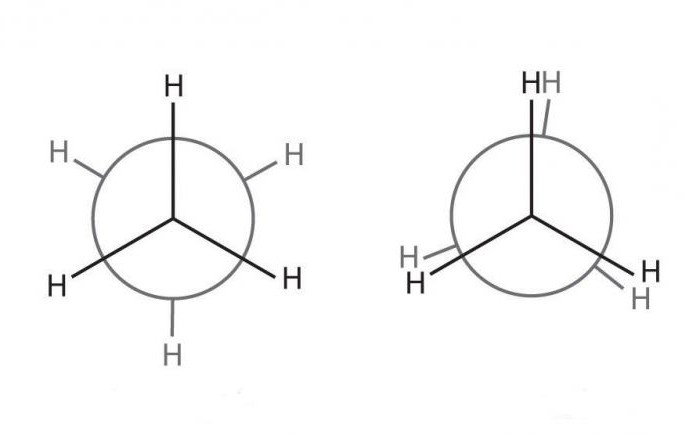
The spatial structure of the ethylene molecule serves as an example of the third variant of the formation of CC bonds, when two tetrahedra have one common face, i.e. intersect at two adjacent vertices. It becomes clear that because of this stereometric position of the molecule, the movement of carbon atoms relative to its axis is difficult, because requires breaking one of the bonds. But it becomes possible the formation of cis and trans isomers of substances, because two free radicals from each carbon can be located either specularly or crosswise.
The cis and transposition of the molecule explains the existence of fumaric and maleic acids. Two bonds are formed between the carbon atoms in these molecules, and each of them has one hydrogen atom and a COOH group.
The last case characterizing the spatial structure of molecules can be represented by two pyramids that have one common face and are interconnected by three vertices. An example is an acetylene molecule.
Firstly, such molecules do not have cis or trans isomers. Secondly, carbon atoms are not able to rotate around its axis. And thirdly, all atoms and their radicals are located on one axis, and the valence angle is 180 degrees.
Of course, the described cases can be applied to substances whose skeleton contains more than two hydrogen atoms. The principle of stereometric construction of such molecules is preserved.
Spatial structure of molecules of inorganic substances
The formation of covalent bonds in inorganic compounds is similar in mechanism to that of organic substances. For the formation of a bond, the presence of lone electron pairs for two atoms, which form a common electron cloud, is necessary.
The overlapping of orbitals during the formation of a covalent bond occurs along one line of the nuclei of atoms. If an atom forms two or more bonds, then the distance between them is characterized by the value of the valence angle.
If we consider a water molecule, which is formed by one oxygen atom and two hydrogen atoms, the valence angle should ideally be 90 degrees. However, experimental studies have proven that this value is 104.5 degrees. The spatial structure of the molecules differs from the theoretically predicted one due to the presence of interaction forces between hydrogen atoms. They repel each other, thereby increasing the valence angle between them.
Sp hybridization
Hybridization is the theory of the formation of identical hybrid orbitals of a molecule. This phenomenon occurs due to the presence of lone electron pairs at different energy levels in the central atom.
As an example, we consider the formation of covalent bonds of the BeCl2 molecule. In Beryllium, unshared electron pairs are at s and p levels, which in theory should serve as the reason for the formation of an uneven angular molecule. However, in practice they are linear, and the valence angle is 180 degrees.
Sp hybridization is used in the formation of two covalent bonds. However, there are other types of formation of hybrid orbitals.
Sp2 hybridization
This type of hybridization is responsible for the spatial structure of molecules with three covalent bonds. An example is the BCl3 molecule. The central barium atom has three lone electron pairs: two at the p level and one at the s level.
Three covalent bonds form a molecule, which is located in one plane, and its valence angle is 120 degrees.
Sp3 hybridization
Another variant of the formation of hybrid orbitals is when the central atom has 4 lone electron pairs: 3 at the p level and 1 at the s level. An example of such a substance is methane. The spatial structure of methane molecules is a tetraerd, in which the valence angle is 109.5 degrees. The change in the angle is characterized by the interaction of hydrogen atoms with each other.