Let's look at how an atom is built. Keep in mind that the discussion will be exclusively about models. In practice, atoms are a much more complex structure. But thanks to modern developments, we are able to explain and even successfully predict the properties of chemical elements (even if not all). So, what is the structure of the atom? What is it made of?
Planetary Atom Model
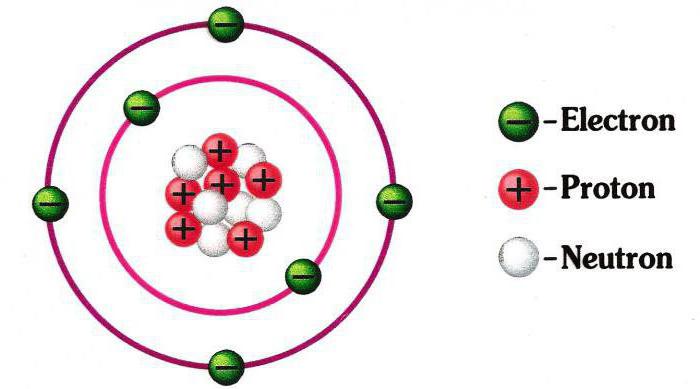
It was first proposed by the Danish physicist N. Bohr in 1913. This is the first theory of atomic structure based on scientific facts. In addition, she laid the foundation for modern thematic terminology. In it, the electron-particles make rotational movements around the atom according to the same principle as the planets around the Sun. Bohr suggested that they can exist exclusively in orbits located at a strictly defined distance from the core. Why exactly, the scientist could not explain from the standpoint of science, but such a model was confirmed by many experiments. To indicate the orbits, integers were used, starting with the unit that was numbered, closest to the core. All of these orbits are also called levels. The hydrogen atom has only one level at which one electron rotates. But complex atoms still have levels. They are divided into components that combine electrons close in energy potential. So, the second one already has two sublevels - 2s and 2p. The third one already has three - 3s, 3p and 3d. And so on. First, sublevels closer to the core are “populated,” and then distant. On each of them can be placed only a certain number of electrons. But this is not the end. Each sublevel is divided into orbitals. Let's make a comparison with ordinary life. The atomic electron cloud is comparable to a city. Levels are streets. Sub-level - private house or apartment. Orbital is a room. In each of them "lives" one or two electrons. All of them have specific addresses. That was the first atomic structure diagram. And finally, about the addresses of electrons: they are determined by sets of numbers, which are called "quantum".
Atom wave model
But over time, the planetary model was revised. A second theory of the structure of the atom was proposed. It is more perfect and allows explaining the results of practical experiments. The first was replaced by the wave model of the atom, which will be proposed by E. Schrodinger. Then it was already established that the electron can manifest itself not only as a particle, but also as a wave. What did Schrödinger do? He applied an equation describing the motion of a wave in
three-dimensional space. Thus, one can find not the trajectory of the electron in the atom, but the probability of its detection at a certain point. What unites both theories is that elementary particles are on specific levels, sublevels and orbitals. This is where the similarity of models ends. I will give one example - in the wave theory the orbital is the region where it will be possible to find an electron with a probability of 95%. The rest of the space accounts for 5%. But in the end it turned out that the structural features of the atoms are depicted using the wave model, while the terminology used is shared.
The concept of probability in this case
Why was this term used? Heisenberg in 1927 formulated the principle of uncertainty, which is now used to describe the movement of microparticles. It is based on their fundamental difference from ordinary physical bodies. What does it consist of? Classical mechanics suggested that a person can observe phenomena without affecting them (observation of celestial bodies). Based on the data obtained, it is possible to calculate where the object will be at a certain point in time. But in the microworld, things must be different. So, for example, observing an electron without affecting it is now not possible due to the fact that the energies of the instrument and particles are not comparable. This leads to the fact that it changes the location of the elementary particle, state, direction, speed and other parameters. And it makes no sense to talk about the exact characteristics. The uncertainty principle itself tells us that it is impossible to calculate the exact path of an electron around a nucleus. You can only indicate the probability of a particle being in a certain area of space. Here is a feature of the structure of atoms of chemical elements. But this should be taken into account exclusively by scientists in practical experiments.
Atom composition
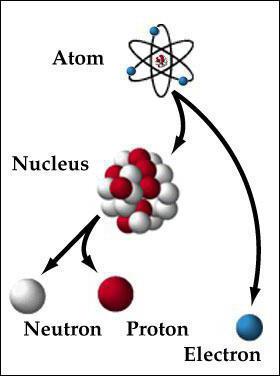
But let's concentrate on the whole object of consideration. So, in addition to the well-considered electron shell, the second component of the atom is the nucleus. It consists of positively charged protons and neutral neutrons. We are all familiar with the periodic table. The number of each element corresponds to the number of protons that it has. The number of neutrons equals the difference between the mass of an atom and its number of protons. There may be deviations from this rule. Then they say that the element isotope is present. The structure of the atom is such that it is "surrounded" by the electron shell. The number of electrons is usually equal to the number of protons. The mass of the latter is approximately 1840 times greater than that of the first, and approximately equal to the weight of the neutron. The radius of the nucleus is about 1/200000 of the diameter of the atom. He himself has a spherical shape. Such, in general, is the structure of atoms of chemical elements. Despite the difference in mass and properties, they look about the same.
Orbits
Speaking of what the structure of the atom is, one cannot remain silent about them. So, there are such types:
- s. They have a spherical shape.
- p. They are similar to volume eights or spindle.
- d and f. They have a complex form, which is hardly described by a formal language.
An electron of each type can be found with a probability of 95% in the territory of the corresponding orbital. The information presented must be treated calmly, because it is more likely an abstract mathematical model than a physical real state of affairs. But with all this, it has good predictive power regarding the chemical properties of atoms and even molecules. The further the level is located from the nucleus, the more electrons can be placed on it. So, the number of orbitals can be calculated using a special formula: x 2 . Here x is equal to the number of levels. And since up to two electrons can be placed on the orbitals, then ultimately the formula for their numerical search will look like this: 2x 2 .
Orbits: technical data
If we talk about the structure of the fluorine atom, then it will have three orbitals. All of them will be filled. The energy of the orbitals within the same sublevel is the same. To indicate them, add the layer number: 2s, 4p, 6d. We return to the conversation about the structure of the fluorine atom. It will have two s- and one p-sublevels. He has nine protons and as many electrons. First one s-level. These are two electrons. Then the second s-level. Two more electrons. And 5 fill the p-level. Here is his structure. After reading the next subheading, you can do the necessary actions yourself and see for yourself. If we talk about the physical properties of halogens, which include fluorine, it should be noted that they, although in the same group, are completely different in their characteristics. So, their boiling point ranges from -188 to 309 degrees Celsius. So why are they combined? All thanks to the chemical properties. All halogens, and to the greatest extent fluorine, have the highest oxidizing ability. They react with metals and without problems can self-ignite at room temperature.
How are orbits filled?
What are the rules and principles of electrons? We offer you to familiarize yourself with the three main ones, the wording of which was simplified for a better understanding:
- The principle of least energy. Electrons tend to fill the orbitals in the order of increasing their energy.
- Pauli principle. No more than two electrons can be located on one orbital.
- Hund rule. Within one sublevel, the electrons fill first the free orbitals, and only then form pairs.
The periodic system of Mendeleev will help in the matter of filling , and the structure of the atom in this case will become more clear in terms of image. Therefore, in practical work with the construction of circuits of elements, it is necessary to keep it at hand.
Example
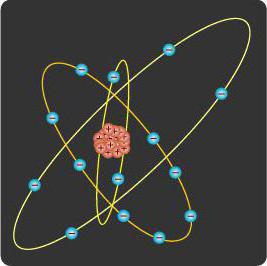
In order to summarize all that has been said in the framework of the article, we can compose a sample of how atomic electrons are distributed over their levels, sublevels and orbitals (that is, what is the configuration of the levels). It can be depicted as a formula, an energy diagram, or as a diagram of layers. Here are very good illustrations, which, upon careful examination, help to understand the structure of the atom. So, first the first level is filled. It has only one sublevel, in which there is only one orbital. All levels are filled sequentially, starting with the smaller. First, within one sublevel, one electron is placed on each orbital. Then couples are created. And in the presence of free there is a switch to another subject of filling. And now you can independently find out what the structure of the nitrogen or fluorine atom is (which was considered earlier). Initially, it can be a little complicated, but you can navigate through the pictures. For clarity, let us consider the structure of the nitrogen atom. It has 7 protons (together with neutrons making up the nucleus) and as many electrons (which make up the electron shell). First, the first s-level is filled. There are 2 electrons on it. Then comes the second s-level. It also has 2 electrons. And the other three are located at the p-level, where each of them occupies one orbital.
Conclusion
As you can see, the structure of the atom is not such a difficult topic (if you approach it from the perspective of a school chemistry course, of course). And to understand this topic is not difficult. Finally, I want to report on some features. For example, speaking about the structure of the oxygen atom, we know that it has eight protons, and 8-10 neutrons. And since everything in nature tends to equilibrium, two oxygen atoms form a molecule, where two unpaired electrons form a covalent bond. Similarly, another persistent oxygen molecule is formed - ozone (O
3 ). Knowing the structure of the oxygen atom, one can correctly formulate the formulas of oxidative reactions in which the most widespread substance on Earth is involved.