Since the end of the worst war in human history, just over two months have passed. And on July 16, 1945, the US military tested the first nuclear bomb, and after another month, thousands of residents of Japanese cities were killed in atomic heat. Since then , nuclear weapons, as well as their means of delivery to targets, have been continuously improved for over half a century.
The military wanted to have at their disposal both heavy-duty ammunition, sweeping entire cities and countries off the map with one blow, and ultra-small ones that fit in a briefcase. Such a device would bring a sabotage war to an unprecedented hitherto level. Insurmountable difficulties arose with both the first and the second. The blame for everything is the so-called critical mass. However, first things first.
Such an explosive core
To understand the order of operation of nuclear devices and understand what is called the critical mass, we will return for a short time to the desk. From the school physics course, we remember a simple rule: charges of the same name repel. In the same place, in high school, students are told about the structure of the atomic nucleus, which consists of neutrons, neutral particles and positively charged protons. But how is this possible? Positive charged particles are located so close to each other, repulsive forces must be colossal.
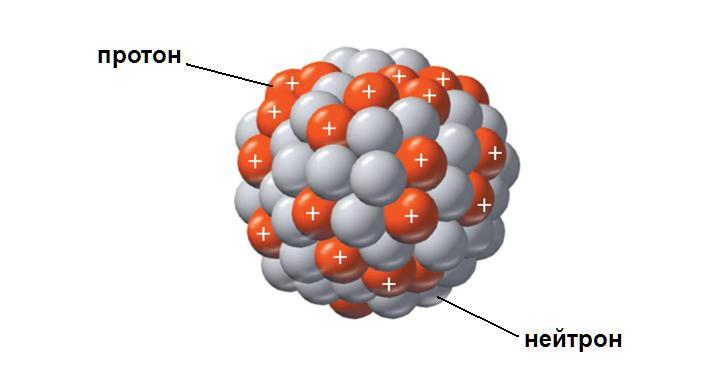
Science is not fully aware of the nature of intranuclear forces holding protons together, although the properties of these forces have been studied quite well. The forces act only at a very close distance. But it is worth at least a little separation of protons in space, as the repulsive forces begin to prevail, and the nucleus shatters into pieces. And the power of such an expansion is truly colossal. It is known that the strength of an adult male would not be enough to hold protons of just one single nucleus of a lead atom.
What frightened Rutherford
The kernels of most elements of the periodic table are stable. However, with increasing atomic number, this stability decreases. It's about the size of the cores. Imagine a nucleus of a uranium atom, consisting of 238 nuclides, of which 92 are protons. Yes, protons are in close contact with each other, and intranuclear forces reliably cement the entire structure. But the repulsive force of protons located at opposite ends of the nucleus becomes noticeable.
What did Rutherford do? He carried out the bombardment of atoms by neutrons (an electron will not pass through the electron shell of an atom, and a positively charged proton will not be able to approach the nucleus due to repulsive forces). A neutron falling into the nucleus of an atom caused its fission. Two separate halves and two or three free neutrons scattered to the sides.
This decay, due to the enormous speeds of flying particles, was accompanied by the release of enormous energy. Rumor had it that Rutherford even wanted to hide his discovery, fearing its possible consequences for humanity, but this is most likely nothing more than a fairy tale.
So what does the mass have to do with and why is it critical
So what? How can a sufficient amount of radioactive metal be irradiated with a stream of protons to produce a powerful explosion? And what is a critical mass? The thing is in those few free electrons that fly out of the "bombed" atomic nucleus; in turn, when they collide with other nuclei, they will cause their fission. The so-called nuclear chain reaction will begin . However, launching it will be extremely difficult.
Clarify the scale. If we take an apple on our table for the nucleus of an atom, then in order to imagine the nucleus of a neighboring atom, we will have to take the same apple and put it on the table not even in the next room, but ... in the next house. The neutron will be the size of a cherry stone.
In order for the emitted neutrons not to be wasted outside the limits of the uranium ingot, and more than 50% of them would find a target in the form of atomic nuclei, this ingot should have the appropriate size. This is what is called the critical mass of uranium - the mass at which more than half of the released neutrons collide with other nuclei.
In fact, this happens in an instant. The number of split nuclei grows like an avalanche, their fragments rush in all directions at speeds comparable to the speed of light, ripping open air, water, any other medium. From their collisions with environmental molecules, the explosion region instantly heats up to millions of degrees, radiating heat that incinerates everything in the vicinity of several kilometers.
The sharply heated air instantly increases in size, creating a powerful shock wave that blows away from the foundations of the building, turns and destroys everything in its path ... such is the picture of an atomic explosion.
What it looks like in practice
The construction of an atomic bomb is surprisingly simple. There are two ingots of uranium (or another radioactive metal), the mass of each of which is slightly less than critical. One of the ingots is made in the form of a cone, the other is a ball with a conical hole. As you might guess, when combining both halves, you get a ball that achieves a critical mass. This is a standard elementary nuclear bomb. Two halves are connected using a regular TNT charge (the cone shoots into a ball).
But do not think that such a device can be assembled "on the knee" by anyone. The trick is that uranium must be very clean for the bomb to explode, the presence of impurities is practically zero.
Why there is no atomic bomb the size of a pack of cigarettes
All for the same reason. The critical mass of the most common isotope of uranium 235 is about 45 kg. The explosion of so much nuclear fuel is already a disaster. And to make an explosive device with less substance is impossible - it just won’t work.
For the same reason, it did not work to create super-powerful atomic charges from uranium or other radioactive metals. In order for the bomb to be very powerful, it was made of a dozen ingots, which, when detonating charges were detonated, rushed to the center, connecting like orange slices.
But what really happened? If for some reason the two elements met a thousandth of a second ahead of the rest, the critical mass was reached faster than the others “arrived”, the explosion did not take place at the power that the designers expected. The problem of heavy-duty nuclear weapons was resolved only with the advent of thermonuclear weapons. But this is a slightly different story.
And how does a peaceful atom work?
A nuclear power plant is essentially the same nuclear bomb. Only at this “bomb” are fuel rods (fuel elements) made of uranium located at a certain distance from each other, which does not prevent them from exchanging neutron “impacts”.
The fuel rods are made in the form of rods, between which there are regulating rods made of a material that absorbs neutrons well. The principle of operation is simple:
- control (absorbing) rods are introduced into the space between the uranium rods - the reaction slows down or stops altogether;
- control rods are removed from the zone - radioactive elements actively exchange neutrons, the nuclear reaction proceeds more intensively.
Indeed, it turns out the same atomic bomb in which the critical mass is achieved so smoothly and regulated so clearly that it does not lead to an explosion, but only to the heating of the coolant.
Although, unfortunately, as practice shows, not always a human genius is able to curb this huge and destructive energy - the energy of the decay of an atomic nucleus.