In 1784, the French military engineer Charles Augustin de Coulomb, who found his true calling in physics, conducted an experiment that went down in the history of science under his name. Coulomb's famous experience laid the foundation for precise quantitative methods for studying various manifestations of electromagnetism.
Event background
Coulomb, who had been fruitfully engaged in science for many years, of course, relied both on the results of research in the field of electricity, obtained by his predecessors, and on his own achievements, which allowed him to succeed in experimentally confirming the already existing ideas about the strength of interaction of electrically charged bodies .
F. Epinus, D. Bernoulli, J. Priestley and other prominent scientists of the second half of the 18th century stated that electric forces obey the inverse quadratic dependence on the distance separating the charges. However, to directly measure these forces before the Coulomb experiment was very difficult.
The reason is that when an external electric field is applied, the bodies are polarized (electrified) - a spatial redistribution of charge density occurs in them, that is, bodies become electric dipoles. As a result, the charges on the bodies under study will interact not only with each other, but also with various elements of the equipment, violating the purity of the experiment, and this interference must be taken into account. In addition, the construction of such a device that would allow measuring small values of electrostatic repulsion or attraction forces was a serious difficulty.
The pendant invented just such a device with sufficient sensitivity - torsion scales. This was possible to him largely due to the fact that the scientist devoted several years to the study of the mechanics of torsion of threads from various materials. It is the amount of twisting of the metal thread of the suspension in the experiment of Coulomb with torsion scales that formed the basis of the experimental setup.
Device device
The main part of the structure is a rocker arm made of shellac suspended from an extremely thin silver wire. At one end of this rocker arm was a gilded ball machined from the core of an elderberry trunk (this material is very dry and light, which contributed to an increase in the sensitivity of the device). The role of the counterweight and stabilizer of the rocker arm was played by a circle of paper impregnated with turpentine fixed on the other end.
The balance was placed in a cylindrical glass vessel; In this case, the wire was passed through an additional narrow upper cylinder, the experimenter equipped the rotary cover with a circular degree scale in order to be able to tell the wire to twist at known angles. The main cylinder was also calibrated around the circumference at the level of the rocker. Its lid had a special hole in which another ball could be inserted on the rod - a copy of the first.
Coulomb experience description
The French physicist conducted the experiment as follows. The pre-electrified ball on the rod was brought into contact with the ball of the rocker arm mounted at the zero mark of the scale. In this case, the electric charge was distributed equally between the balls, since they had equal diameters and, correspondingly, surface areas.
Due to the electrostatic repulsion of the balls, the beam turned, spinning the silver thread through an angle, the magnitude of which depends on the repulsive force and on the elasticity of the thread, which Coulomb knew well. Then the experimenter gave the wire a reverse twist and again fixed the deflection angles of the rocker arm on a scale on a large cylinder. The scientist determined the dependence of force on the distance between charges by the ratio between the angle of deviation of the beam from the zero position and the total angle of twisting (taking into account the twisting of the thread).
In the experiment, Coulomb managed to find out the relationship between the repulsive force and the magnitude of the electric charge by successively dividing the charge of the electrified ball in half by contact with a neutral ball of the same size on the insulating handle.
The establishment of a physical law
The device gave a rather large error for a number of reasons: charge leakage, the inability to set the rocker to exactly zero scales, measuring the distance in the corners, and not directly between the charges, and so on. The pendant, however, was able to evaluate and take into account this error and accurately establish the relationship between electrostatic forces, the magnitude of the charges and the distance between them. The Coulomb law established in a series of experiments in the formulation of the scientist himself states: the modulus of force with which two point electric charges interact in a vacuum is proportional to the product of their values and inversely proportional to the square of the distance by which they are separated from each other. The mathematical form of this dependence is as follows: F ~ (q 1 ∙ q 2 ) / r 2 .
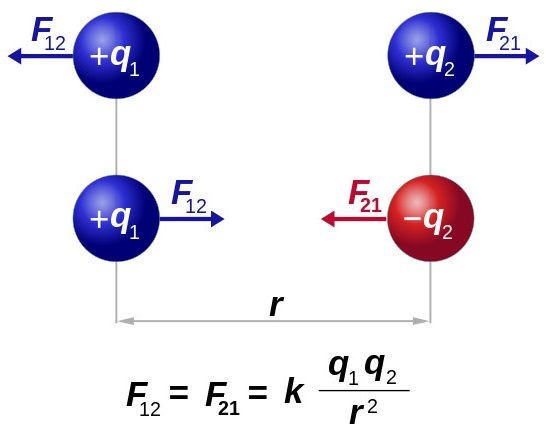
The modern definition only adds that the force acts along a straight line that connects the charges. In addition, the dependence turns into an equation by introducing a proportionality coefficient associated with the electric constant: F = k (q 1 ∙ q 2 ) / r 2 .
Coulomb's experience was of great importance to physics. For the first time, he allowed not only to reveal one of the fundamental laws of electromagnetism, but also to give him a clear mathematical formulation, which made it possible to quantitatively describe an extensive class of natural phenomena.