Mutual transformations of compounds observed in living nature, as well as occurring as a result of human activity, can be considered as chemical processes. The reagents in them can be either two or a larger number of substances in one or in different states of aggregation. Depending on this, homogeneous or heterogeneous systems are distinguished. The conditions, features of the course and the role of chemical processes in nature will be considered in this paper.
What is meant by a chemical reaction
If, as a result of the interaction of the starting materials, the constituent parts of their molecules are changed, and the charges of the nuclei of the atoms remain the same, they speak of chemical reactions or processes. Products formed as a result of their flow are used by man in industry, agriculture and everyday life. A huge number of interactions between substances occur, both in living and inanimate nature. Chemical processes have a fundamental difference from physical phenomena and the properties of radioactivity. Molecules of new substances are formed in them, while physical processes do not change the composition of compounds, and atoms of new chemical elements appear in nuclear reactions.
Conditions for the implementation of processes in chemistry
They can be different and depend, first of all, on the nature of the reagents, the need for an influx of energy from the outside, as well as the state of aggregation (solids, solutions, gases) in which the process occurs. The chemical mechanism of interaction between two or more compounds can be carried out under the influence of catalysts (for example, production of nitric acid), temperature (ammonia production), and light energy (photosynthesis). With the participation of enzymes in wildlife, the processes of the chemical reaction of fermentation (alcohol, lactic acid, butyric acid) used in the food and microbiological industry are widespread. To obtain products in the organic synthesis industry, one of the main conditions is the presence of a free-radical mechanism of the chemical process. An example would be the production of chlorine derivatives of methane (dichloromethane, trichloromethane, carbon tetrachloride, resulting from chain reactions.
Homogeneous Catalysis
They are special types of contact of two or more substances. The essence of chemical processes occurring in a homogeneous phase (for example, gas - gas) involving reaction accelerators consists in carrying out reactions in the entire volume of mixtures. If the catalyst is in the same state of aggregation as the reactants, it forms mobile intermediate complexes with the starting compounds.
Homogeneous catalysis is the main chemical process carried out, for example, in oil refining, in the production of gasoline, naphtha, gas oil, and other types of fuel. It uses technologies such as reforming, isomerization, catalytic cracking.
Heterogeneous catalysis
In the case of heterogeneous catalysis, contact of the reacting substances occurs, most often, on the solid surface of the catalyst itself. So-called active centers are formed on it. These are areas in which the interaction of reacting compounds proceeds very quickly, that is, the reaction rate is high. They are species-specific and play an important role also if chemical processes occur in living cells. Then they talk about metabolism - metabolic reactions. An example of heterogeneous catalysis is the industrial production of sulfate acid. In the contact apparatus, a gaseous mixture of sulfur dioxide and oxygen is heated and passed through trellised shelves filled with dispersed powder of vanadium oxide or vanadyl sulfate VOSO 4 . The resulting product is sulfur trioxide, then it is absorbed by concentrated sulfuric acid. A liquid called oleum is formed. It can be diluted with water to obtain the desired concentration of sulfate acid.
Features of thermochemical reactions
The release or absorption of energy in the form of heat is of great practical importance. It is enough to recall the reaction of fuel combustion: natural gas, coal, peat. They are physicochemical processes, an important characteristic of which is the calorific value. Thermal reactions are widespread both in the organic world and in inanimate nature. For example, in the process of digestion, the breakdown of proteins, lipids and carbohydrates occurs under the action of biologically active substances - enzymes.

The released energy is accumulated in the form of macroergic bonds of ATP molecules. Dissimilation reactions are accompanied by the release of energy, part of which is dissipated in the form of heat. As a result of digestion, each gram of protein gives 17, 2 kJ of energy, starch - 17, 2 kJ, fat - 38.9 kJ. Chemical processes that occur with the release of energy are called exothermic, and with its absorption, endothermic. In the organic synthesis industry and other technologies, the thermal effects of thermochemical reactions are calculated. This is important to know, for example, in order to correctly calculate the amount of energy used to heat the reactors and synthesis columns in which reactions accompanied by absorption of heat occur.
Kinetics and its role in the theory of chemical processes
The calculation of the speed of reacting particles (molecules, ions) is the most important task facing the industry. Its solution provides the economic effect and profitability of technological cycles in chemical production. To increase the rate of such a reaction, such as ammonia synthesis, the decisive factors will be a change in pressure in the gas mixture of nitrogen and hydrogen up to 30 MPa, as well as preventing a sharp increase in temperature (the optimum temperature is 450-550 ° C).
The chemical processes used in the production of sulfate acid, namely: burning pyrites, oxidation of sulfur dioxide, absorption of sulfur trioxide by oleum is carried out under various conditions. For this, a pyrite furnace and contact devices are used. They take into account the concentration of reacting substances, temperature and pressure. All these factors are correlated for the reaction at the highest rate, which increases the yield of sulfate acid to 96-98%.
The cycle of substances as physical and chemical processes in nature
The well-known saying “Motion is life” can be applied to chemical elements that enter into various types of interaction (reaction of compound, substitution, decomposition, exchange). Molecules and atoms of chemical elements arrive in continuous motion. As scientists have established, all of the above types of chemical reactions can be accompanied by physical phenomena: the generation of heat or its absorption, the emission of photons of light, a change in the state of aggregation. These processes occur in every shell of the Earth: lithosphere, hydrosphere, atmosphere, biosphere. The most significant of these are the cycles of substances such as oxygen, carbon dioxide and nitrogen. In the next heading, we will examine how nitrogen circulates in the atmosphere, soil, and living organisms.
Interconversion of nitrogen and its compounds
It is well known that nitrogen is a necessary component of proteins, which means that it is involved in the formation of all types of earthly life, without exception. Nitrogen is absorbed by plants and animals in the form of ions: ammonium, nitrate and nitrite ions. As a result of photosynthesis, plants form not only glucose, but also amino acids, glycerin, fatty acids. All of the above chemical compounds are products of reactions occurring in the Calvin cycle. The outstanding Russian scientist K. Timiryazev spoke about the cosmic role of green plants, bearing in mind, among other things, their ability to synthesize proteins.
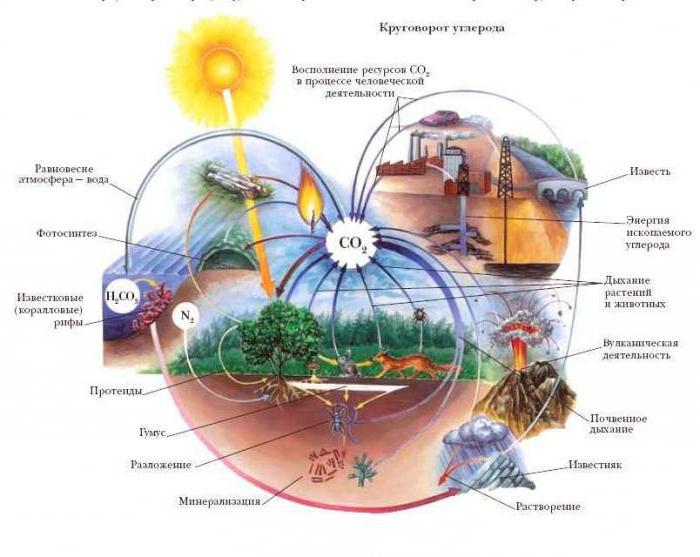
Herbivores receive peptides from plant foods, and carnivores from victims' meat. During the decay of plant and animal remains under the influence of saprotrophic soil bacteria, complex biological and chemical processes occur. As a result, nitrogen from organic compounds goes into inorganic form (ammonia, free nitrogen, nitrates and nitrites are formed). Returning to the atmosphere and soil, all these substances are again absorbed by plants. Nitrogen enters through the stomata of the skin of the leaves, and solutions of nitric and nitrous acids and their salts are absorbed by the root hairs of the plant roots. The nitrogen conversion cycle is closed to repeat again. The essence of chemical processes occurring with nitrogen compounds in nature was studied in detail at the beginning of the 20th century by the Russian scientist D.N. Pryanishnikov.
Powder Metallurgy
Modern chemical processes and technologies make a significant contribution to the creation of materials with unique physical and chemical properties. This is especially important, first of all, for instruments and equipment of oil refineries, enterprises producing inorganic acids, dyes, varnishes, plastics. Heat exchangers, contact devices, synthesis columns, pipelines are used in their production. The surface of the equipment is in contact with aggressive media under high pressure. Moreover, almost all chemical production processes are carried out under the influence of high temperatures. It is relevant to obtain materials with high thermal and acid resistance, anti-corrosion properties.
Powder metallurgy includes the processes of production of metal-containing powders, sintering and introduction into the composition of modern alloys used in reactions with chemically aggressive substances.
Composites and their meaning
Among modern technologies, the most important chemical processes are reactions to obtain composite materials. These include foams, cermets, norpapalsts. As a matrix for production, metals and their alloys, ceramics, and plastics are used. As fillers, calcium silicate, white clay, strontium and barium ferrides are used. All of the above substances give composite materials impact resistance, heat and wear resistance.
What is chemical technology
The branch of science that studies the means and methods used in the reactions of processing raw materials: oil, natural gas, coal, minerals, was called chemical technology. In other words, it is a science of chemical processes that occur as a result of human activity. Its entire theoretical base is made up of mathematics, cybernetics, physical chemistry, and industrial economics. It doesn’t matter which chemical process is involved in the technology (production of nitrate acid, limestone decomposition, synthesis of phenol-formaldehyde plastics) - in modern conditions it is impossible without automated control systems that facilitate human activities, eliminate environmental pollution, and provide continuous and waste-free technology for chemical production.
In this paper, we examined examples of chemical processes that occur both in living nature (photosynthesis, dissimilation, nitrogen cycle), and in industry.