Carbolic acid is one of the names of phenol, indicating its special behavior in chemical processes. This substance is lighter than benzene undergoes nucleophilic substitution reactions. The acid properties inherent in the compound are explained by the mobility of the hydrogen atom in the hydroxyl group bonded to the ring. Studying the structure of the molecule and qualitative reactions to phenol make it possible to attribute the substance to aromatic compounds - benzene derivatives.
Phenol (hydroxybenzene)
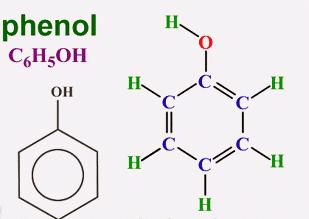
In 1834, the German chemist Runge isolated carbolic acid from coal tar, but was unable to decipher its composition. Later, other researchers proposed a formula and attributed the new compound to aromatic alcohols. The simplest representative of this group is phenol (hydroxybenzene). In its pure form, this substance is a transparent crystal with a characteristic odor. In air, the color of phenol can change, turn pink or red. Aromatic alcohol is characterized by poor solubility in cold water and good in organic solvents. Phenol melts at a temperature of 43 ° C. It is a toxic compound and, on contact with the skin, causes severe burns. The aromatic part of the molecule is represented by the phenyl radical (C6H5—). Hydroxyl group oxygen (—OH) is bonded directly to one of the carbon atoms. The presence of each of the particles proves the corresponding qualitative reaction to phenol. The formula showing the total atomic content of chemical elements in the molecule is C6H6O. The structure is reflected in structural formulas including the Kekule cycle and the hydroxyl functional group. Spherical rod models give a clear idea of the molecule of aromatic alcohol.
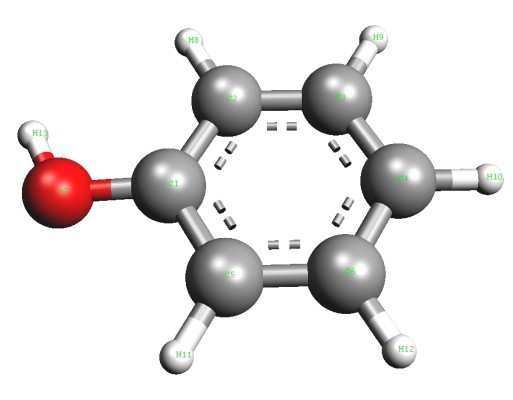
Molecular Structure Features
The interaction of the benzene core and the OH group determines the chemical reactions of phenol with metals, halogens, and other substances. The presence of an oxygen atom associated with the aromatic cycle leads to a redistribution of electron density in the molecule. The O – H bond becomes more polar, which leads to an increase in the mobility of hydrogen in the hydroxyl group. The proton can be replaced by metal atoms, which indicates the acidity of phenol. In turn, the OH group increases the reaction properties of the benzene ring. The delocalization of electrons and the ability to electrophilic substitution in the nucleus increases. In this case, the mobility of hydrogen atoms bonded to carbon in the ortho and para position increases (2, 4, 6). This effect is due to the presence of an electron density donor - a hydroxyl group. Due to its influence, phenol is more active than benzene in reactions with certain substances, and new substituents are oriented in ortho and para positions.
Acid properties
In the hydroxyl group of aromatic alcohols, the oxygen atom acquires a positive charge, weakening its bond with hydrogen. Proton release is facilitated, so phenol behaves like a weak acid, but stronger than alcohols. Qualitative reactions to phenol include a litmus test, which in the presence of protons changes color from blue to pink. The presence of halogen atoms or nitro groups bonded to the benzene ring leads to an increase in hydrogen activity. The effect is observed in the molecules of nitro derivatives of phenol. Substituents such as amino and alkyl (CH3—, C2H5— and others) reduce acidity. The compounds combining in its composition a benzene ring, a hydroxyl group and a methyl radical include cresol. Its properties are weaker than carbolic acid.
The reaction of phenol with sodium and alkali
Like acids, phenol reacts with metals. For example, it reacts with sodium: 2C6H5 — OH + 2Na = 2C6H5 — ONa + H2 ↑.
Sodium phenolate forms and hydrogen gas is released. Phenol interacts with soluble bases. A
neutralization reaction occurs with the formation of salt and water: C6H5 — OH + NaOH = C6H5 — ONa + H2O. The ability to give hydrogen in the hydroxyl group of phenol is lower than that of most inorganic and carboxylic acids. Even carbon dioxide (carbonic acid) dissolved in water displaces it from salts. Reaction equation: C6H5 — ONa + CO2 + H2O = C6H5 — OH + NaHCO3.
Benzene ring reactions
Aromatic properties are due to the delocalization of electrons in the benzene core. Hydrogen from the ring is replaced by halogen atoms, a nitro group. A similar process in the phenol molecule is easier than in benzene. One example is bromination. Halogen acts on benzene in the presence of a catalyst, resulting in bromobenzene. Phenol reacts with bromine water under normal conditions. As a result of the interaction, a white precipitate of 2,4,6-tribromophenol is formed, the appearance of which allows us to distinguish the test substance from similar aromatic compounds. Bromination is a qualitative reaction to phenol. Equation: C6H5 — OH + 3Br2 = C6H2Br3 + HBr. The second reaction product is hydrogen bromide. When phenol reacts with dilute nitric acid , nitro derivatives are obtained. The reaction product with concentrated nitric acid - 2,4,6-trinitrophenol or picric acid is of great practical importance.
Qualitative reactions to phenol. List
The interaction of substances produces certain products that allow you to establish the qualitative composition of the starting materials. A number of color reactions indicate the presence of particles, functional groups, which is convenient to use for chemical analysis. Qualitative reactions to phenol prove the presence of an aromatic ring substance and an OH group in the molecule:
- In a phenol solution, the blue litmus test turns red.
- Color reactions to phenols are also carried out in a weak alkaline medium with diazonium salts. Yellow or orange azo dyes are formed.
- Reacts with bromine brown water, a white precipitate of tribromophenol appears.
- As a result of the reaction with a solution of iron chloride, ferric phenoxide is obtained - a substance of blue, violet or green color.
Getting phenols
Phenol production in industry proceeds in two or three stages. At the first stage, cumene (the trivial name for isopropylbenzene) is obtained from propylene and benzene in the presence of aluminum chloride . Friedel-Crafts reaction equation: C6H5 — OH + C3H6 = C9H12 (cumene). Benzene and propylene in a ratio of 3: 1 are passed over an acid catalyst. More and more often instead of the traditional catalyst - aluminum chloride - environmentally friendly zeolites are used. At the final stage, oxygen oxidation is carried out in the presence of sulfuric acid: C6H5 — C3H7 + O2 = C6H5 — OH + C3H6O. Phenols can be obtained from coal by distillation, are intermediate compounds in the production of other organic substances.
The use of phenols
Aromatic alcohols are widely used in the production of plastics, dyes, pesticides and other substances. The production of carbolic acid from benzene is the first step in creating a number of polymers, including polycarbonates. Phenol reacts with formaldehyde to produce phenol formaldehyde resins.
Cyclohexanol serves as a raw material for the production of polyamides. Phenols are used as antiseptics and disinfectants in deodorants, lotions. Used to produce phenacetin, salicylic acid and other drugs. Phenols are used in the production of resins, which are used in electrical products (switches, sockets). They are also used in the preparation of azo dyes, such as phenylamine (aniline). Picric acid, which is a nitro derivative of phenol, is used for dyeing fabrics, making explosives.