Upon receipt of various types of alkylbenzenes and acylbenzenes in industry, the Friedel – Crafts reaction was widely used. It is one of two known methods for the synthesis of these compounds, and its parameters are regulated to achieve a higher yield.
More on arena alkylation processes
The most famous example of the Friedel – Crafts reaction is the interaction of methyl chloride (CH 3 Cl) with benzene (C 6 H 6 ) in the presence of aluminum chloride (AlCl 3 ), where toluene (C 7 H 9 ) is obtained. This reaction was received in 1877 by two scientists - Charles Friedel and James Crafts. It subsequently became one of the important components for the industrial production of alkylarenes.
The main synthesis is the interaction of benzene and its homologs with any alkyl halides in the presence of the so-called Lewis acids. The essence of a change in reagents does not change: the reaction always follows the same principle. The derivatives of this method was the production in organic chemistry of alkylbenzenes by the interaction of alcohol and an inorganic acid, a carbonium ion and an aromatic ring.
The second method is considered to be the method of converting the side chain of various aromatic ketones in the presence of zinc amalgam (ZnHg) with hydrochloric acid (HCl) or hydrazine (N 2 H 2 ) with a strong base. Both reactions are reducing in nature: the first is called the Clemens reaction, the second - according to Kizhner Wolf.
Also, if unsaturated bonds are present in the side chain, they can be reduced by reaction on a nickel catalyst (Ni) in the presence of hydrogen gas (H 2 ).
Reaction mechanisms
Two possible pathways of the reaction are described in the literature, and both of them follow the principle of electrophilic substitution. The difference lies only in the nature of the electrophile: in the first case, it is an alkyl carbonium ion (another name is carbocation), formed as a result of the addition of the halogen ion to the Lewis acid according to the donor-acceptor principle, and in the second, the one-stage creation of an internal complex between all the reactants involved in the same way. Each of the possible options is discussed in detail below.
The reaction with the formation of a carbonium ion
This mechanism involves the synthesis in 3 stages, where Lewis acids, for example AlCl 3 , TiCl 4 , SnCl 4 , FeCl 3 , BF 3 , HF act as a catalyst for the process.
To consider a typical Friedel-Crafts reaction, the interaction between benzene and 1-fluoropropane (C 3 H 6 F) in the presence of boron trifluoride BF 3 as a catalyst was chosen.
In the first stage of the process, C 3 H 6 ‒F reacts with BF 3 by adding a halogen ion according to the donor-acceptor principle. At the external energy level , boron has a free cell (acceptor), which is occupied by fluorine with a lone pair of electrons (donor). Due to this addition, the carbon atom C, which is adjacent to the halogen F in 1-fluoropropane, acquires a positive charge and becomes a very reactive propyl-carbonium ion. This property of these ions increases in the series primary → secondary → tertiary; therefore, depending on the conditions in the products of the alkylation reaction, the side chain may rearrange to a more favorable position.
Further, the obtained carbocation cation reacts with benzene and is attached at the bond site of carbon and hydrogen atoms, transferring the electron density to the C of the aromatic ring.
In the third stage, the resulting particle reacts with ionized Lewis acid, where the H atom splits off from the arena and joins the detached F to form hydrogen fluoride HF, and n-propylbenzene, isopropylbenzene and reduced BF 3 become the reaction products.
Synthesis with the formation of an internal complex
The reaction mechanism involves the formation of an intermediate common complex, where in one step the alkyl group moves from the halogen to the aromatic ring, and the halogen to the Lewis acid, creating an ion pair that decomposes into alkylbenzene, a mineral compound and a reduced catalyst.
Types of Derivative Reactions
The Friedel – Crafts reaction for benzene and its homologues with alcohols in the presence of mineral acids proceeds according to the same mechanisms. In this case, the hydrogen atom is attached to the hydroxide ion and, detaching itself, forms a water molecule. The resulting carbonium ion is attached to carbon in the aromatic ring at the site of bond with H. This atom is cleaved off by attaching to an acid residue, and as a result, alkylbenzene is synthesized.
In unsaturated hydrocarbons, the detached hydrogen rises at the site of the double bond, forming the same carbocation, bound to the acid residue. Hydrogenation of alkene takes place near the carbon atom, which forms the most favorable structure. Then the reaction proceeds as in the previous case.
A derivative of the syntheses is also the Friedel-Crafts acylation reaction, where instead of alkyl halides, acid chlorides (RCOCl) are used to form aromatic ketones.
The addition of two or more alkyl residues
In the Friedel-Crafts reaction, benzene can attach from 2 to 6 substituents. It should be noted that each time the interaction is faster, since the bond in the aromatic ring is weakened already during the first synthesis. The process of formation of polyalkylbenzenes can be carried out in a single reaction, therefore, to control the receipt of the desired product, an excess of aromatic compounds is used. Using this method, one group can be gradually introduced into the structure of benzene and its homologues.
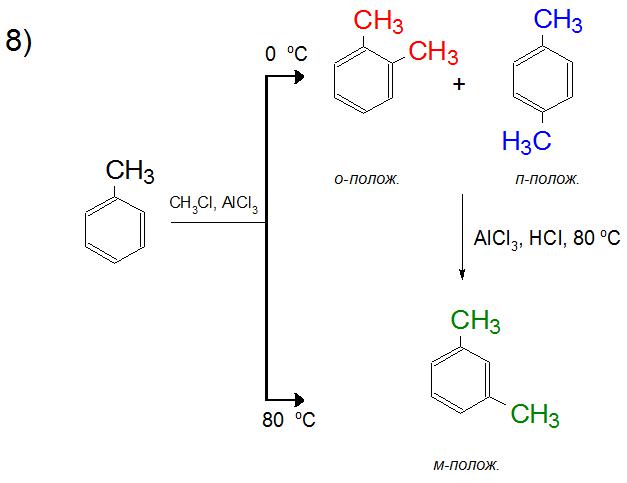
In the Friedel-Crafts reaction, toluene readily attaches the following alkyl group, since arenes have already been activated with respect to electrophilic substitution. In the reaction products at 0 ° C there will be an equilibrium mixture of ortho- and para-xylene, and when the temperature rises to 80 ° C, only a meta-compound will be synthesized. This is explained, as will be described below, by the energy benefit of the formation of certain positions depending on the heating of the mixture.
A continuation of this synthesis is the possible ability of polyhaloalkanes to attach more than one aromatic ring according to the basic mechanism.
Synthesis Features
In organic chemistry, the formation of a mixture of alkylbenzene isomers is explained by two reasons. Firstly, as was said above, the formation of carbocation sometimes suggests a more favorable rearrangement, due to which various product structures are formed. Secondly, their quantitative composition is regulated by the temperature regime (from 0 ° C to 80 ° C), that is, with increasing temperature in order to compensate for the energy consumption of the formation of a specific structure, a higher yield of one of the isomers can be achieved. The same principle applies to the formation of dialkylbenzenes, where the ortho and para positions with increasing temperature are inferior to the meta orientation.
Restrictions on the use of synthesis
There are 3 nuances due to which the Friedel ‒ Crafts reaction can go with side effects or not go at all.
The introduction of electrodeficient substituents into the aromatic ring is accompanied by deactivation of the arena with respect to further substitution reactions. So, for example, when nitronium ion is attached to alkylbenzenes, the synthesis is more difficult, since it pulls the electron density onto itself due to the tendency of nitrogen to fill an empty cell at the external energy level. For the same reasons, polynitration or, for example, polysulfonation takes place under very severe conditions, since with each subsequent synthesis the aromatic ring loses its reactivity.
Therefore, Friedel-Crafts synthesis does not occur if there are electro-deficient substituents in the aromatic ring, especially those having strongly basic properties that bind Lewis acids (for example, -NH 2 , -NHR, -NR 2 ). But the reaction, for example, with halogenbenzenes or aromatic carboxylic acids proceeds according to the typical mechanism, although they have less reactivity.
An important point is also the rearrangement of the carbonium ion in the process or the product at the end, since it is greatly influenced by the synthesis conditions, in particular, the temperature and excess of the alkylated substance.
Instead of the alkyl halides R ‒ X (R = alkyl group, X = halogen), halogenarenes Ar ‒ X (Ar = aromatic compound) cannot be used, since they are very difficult to undergo cleavage of the substituent even under the influence of Lewis acids.