With the development of the study of organic chemistry among a large group of hydrocarbons, a separate class was identified - “alkynes”. These compounds are commonly called unsaturated hydrocarbons that contain one or more triple (other names are triple carbon ‒ carbon or acetylene) bonds in their structure, which distinguishes them from alkenes (compounds with double bonds).
In various sources you can find a rational common name for alkynes - acetylene hydrocarbons, and the same name for their "residues" - acetylene radicals. The alkynes in the table below are presented with their structural formula and various names.
The simplest representatives of alkynes| Structural formulas | Nomenclature |
| International IUPAC | Rational |
| HC ≡ CH | ethine, acetylene | acetylene |
| H 3 C - C ≡ CH | propine | methylacetylene |
| H 3 C - CH 2 - C≡CH | butin-1 | ethylacetylene |
| H 3 C - C≡C - CH 3 | butin-2 | dimethylacetylene |
| H 3 C - CH 2 - CH 2 - C ≡ CH | pentin-1 | propylacetylene |
| H 3 C - CH 2 - C ≡ C - CH 3 | pentin-2 | methylethylacetylene |
International and rational nomenclature
Alkines in chemistry, according to the IUPAC nomenclature (transliteration from the English International Union of Theoretical and Applied Chemistry), are given names by changing the suffix "-an" to the suffix "-in" in the name of a related alkane, for example ethane → ethine (example 1).
But rational names can also be used, for example: ethine → acetylene, propine → methylacetylene (example 2), that is, add the name of the radical located near the triple bond to the name of a smaller representative of the homologous series.
It should be remembered that when determining the name of complex substances, where there are double and triple bonds, the numbering should be such that they get the lowest numbers. If there is a choice between the start of numbering, then start with double bonds, for example: pentene-1, -in-4 (example 3).
A special case of this rule is double and triple bonds equidistant from the end of the chain, as, for example, in the hexadiene-1,3, -in-5 molecule (example 4). It should be remembered that the numbering of the chain will begin with a double bond.
For alkynes with a long chain of links (more than C 5 -C 6 ), it is recommended to use the international IUPAC nomenclature.
Triple bond molecule structure
The most common example of the structure of an acetylene hydrocarbon molecule is presented for ethine, the structure of which can be seen in the table of alkynes. For ease of understanding, a detailed drawing of the interaction of carbon atoms in an acetylene molecule will be given below.
The general formula of alkyne is C 2 H 2 . Therefore, 2 carbon atoms are involved in the process of creating a triple bond. Since carbon is tetravalent — the excited state of an atom — in organic compounds, there are 4 unpaired electrons on the outer orbital - 2s and 2p 3 (Fig. 1a). In the process of creating a connection, a hybrid cloud is formed from the electron clouds of the s- and one p-orbitals, which is called the sp-hybrid cloud (Fig. 1b). Hybrid clouds in both carbon atoms are strongly oriented along one axis, which determines their linear arrangement (at an angle of 180 °) relative to each other in smaller parts outward (Fig. 2). Electrons in most parts of the cloud, when connected, form an electron pair and create a σ-bond (sigma-bond, Fig. 1c).
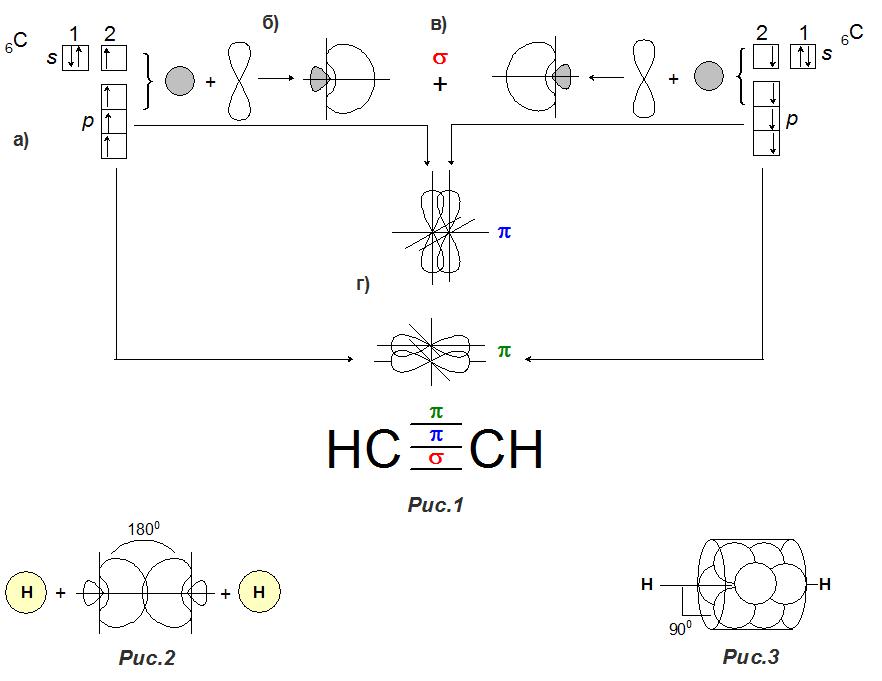
An unpaired electron located in the smaller part attaches the same electron to the hydrogen atom (Fig. 2). The remaining 2 unpaired electrons on the outer p-orbit of one atom interact with 2 other same electrons of the second atom. In this case, each pair of two p-orbitals overlaps according to the principle of π-coupling (pi-coupling, Fig. 1d) and becomes oriented relative to the other at an angle of 90 °. After all interactions, the common cloud takes a cylindrical shape (Fig. 3).
Physical properties of alkynes
Acetylene hydrocarbons are very close in nature to alkanes and alkenes. In nature, they practically do not occur, except ethine, so they are obtained artificially. Lower alkynes (up to C 17 ) are colorless gases and liquids. These are low-polar substances, as a result of which they are poorly soluble in water and other polar solvents. However, they dissolve well in simple organic substances such as ether, naphtha or benzene, and with the increase in pressure during gas compression this ability improves. The highest representatives of this class (C 17 and above) are crystalline substances.
More on acetylene properties
Since acetylene is the most used and widely used, the physical properties of alkyne are well understood. It is a colorless gas with an absolute odorless chemical purity. Technical ethine has a pungent odor due to the presence of impurities of ammonia NH 3 , hydrogen sulfide H 2 S and hydrogen fluoride HF. This liquefied or gaseous gas is very explosive and easily combustible even from static discharge of fingers. Also, due to its physical properties, alkine in a mixture with oxygen gives a combustion temperature of 3150 ° C, which allows the use of acetylene as a good combustible gas in welding and cutting of metals. Acetylene is toxic, so extreme care must be taken when working with this gas.
Acetylene is best dissolved in acetone, especially in a liquefied state, therefore, when stored in a liquid state, special cylinders filled with a porous mass with uniformly distributed acetone under a pressure of up to 25 MPa are used.
And when used in a gaseous state, gas is released through special pipelines, guided by regulatory and technical documentation and GOST 5457-75 “Dissolved and gaseous technical acetylene. Technical conditions ”, which describes the formula of alkyne and all procedures for checking and storing the above gaseous and liquid hydrocarbons.
Acetylene Production
One of the methods is the partial thermal oxidation of methane CH 4 with oxygen at a temperature of 1500 ° C. This process is also called thermal oxidative cracking. A practically similar process occurs when methane is oxidized on an electric arc at a temperature above 1500 ° C with rapid cooling of the evolved gases, because due to the physical properties of alkyn, acetylene in a mixture with unreacted methane can provoke an explosion. Also, this product can be obtained by reacting calcium carbide CaC 2 and water at 2000 ° C.
Application
Among the homologues, as described above, only acetylene received large-scale and constant use, and historically, it was the rational name used in production.
Due to its physical and chemical properties and the relatively cheap way to obtain this hydrocarbon is used in the production of various organic solvents, synthetic rubbers and polymers.