Why can atoms combine with each other and form molecules? What is the reason for the possible existence of substances that include atoms of completely different chemical elements? These are global issues affecting the fundamental concepts of modern physical and chemical science. You can answer them, having an idea of the electronic structure of atoms and knowing the characteristics of the covalent bond, which is the basic basis for most classes of compounds. The purpose of our article is to familiarize yourself with the mechanisms of formation of various types of chemical bonds and the properties of the compounds containing them in their molecules.
The electronic structure of the atom
Electrically neutral particles of matter, which are its structural elements, have a structure that reflects the structure of the solar system. As the planets revolve around the central star - the Sun, so the electrons in the atom move around a positively charged nucleus. To characterize the covalent bond, the electrons located at the last energy level and farthest from the nucleus will be significant. Since their connection with the center of their own atom is minimal, they are easily attracted to the nuclei of other atoms. This is very important for the appearance of interatomic interactions leading to the formation of molecules. Why is it that the molecular form is the main mode of existence of matter on our planet? Let's figure it out.
The main property of atoms
The ability of electrically neutral particles to interact, leading to a gain in energy, is their most important feature. Indeed, under ordinary conditions, the molecular state of a substance is more stable than an atomic one. The basic principles of modern atomic-molecular theory explain both the principles of the formation of molecules and the characteristics of covalent bonds. Recall that at the external energy level of an atom there can be from 1 to 8 electrons, in the latter case the layer will be completed, and therefore very stable. This structure of the external level has atoms of noble gases: argon, krypton, xenon - inert elements that complete each period in the system of D. I. Mendeleev. The exception here will be helium, in which at the last level there are not 8, but only 2 electrons. The reason is simple: in the first period there are only two elements whose atoms have a single electron layer. All other chemical elements have from 1 to 7 electrons on the last, incomplete layer. In the process of interaction between themselves, the atoms will tend to fill with electrons to an octet and restore the configuration of the atom of an inert element. This state can be achieved in two ways: by the loss of one's own or by the adoption of other people's negatively charged particles. These forms of interaction explain how to determine which bond — ionic or covalent — will arise between the atoms that enter into the reaction.
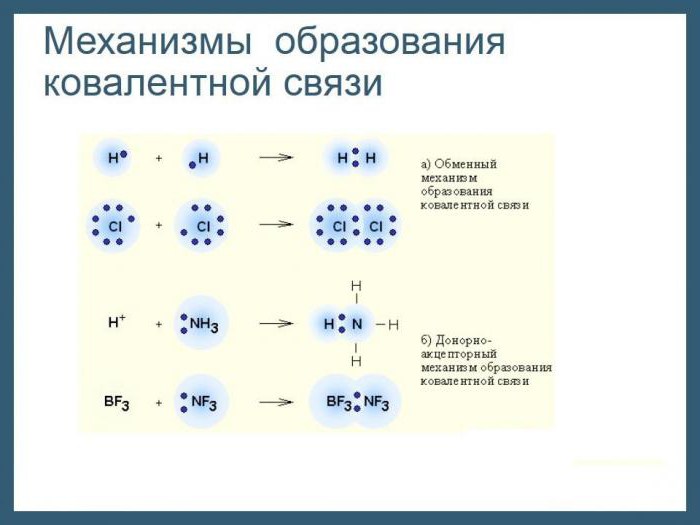
Mechanisms of formation of a stable electronic configuration
Imagine that two simple substances enter into the reaction of a compound: metallic sodium and chlorine gas. A salt class substance is formed - sodium chloride. It has an ionic type of chemical bond. Why and how did it arise? Let us again turn to the structure of the atoms of the starting materials. In sodium, only one electron is located on the last layer, weakly bonded to the nucleus due to the large atomic radius. The ionization energy of all alkali metals, which include sodium, is low. Therefore, the electron of the outer level leaves the energy level, is attracted by the nucleus of the chlorine atom and remains in its space. This creates a precedent for the transition of the Cl atom to the form of a negatively charged ion. Now we are no longer dealing with electrically neutral particles, but with charged sodium cations and chlorine anions. In accordance with the laws of physics, electrostatic attraction forces arise between them, and the compound forms an ionic crystal lattice. The mechanism of formation of an ionic type of chemical bond that we have examined will help to more clearly clarify the specifics and basic characteristics of covalent bonds.
Common electronic pairs
If an ionic bond arises between the atoms of elements that are very different in electronegativity, i.e., metals and nonmetals, then the covalent type appears during the interaction of atoms of the same and different nonmetallic elements. In the first case, it is customary to talk about non-polar, and in the other, about the polar form of covalent bond. The mechanism of their formation is general: each of the atoms partially gives up for use by the electrons, which are combined in pairs. But the spatial arrangement of electron pairs relative to the nuclei of atoms will be different. On this basis, and distinguish between types of covalent bonds - non-polar and polar. Most often, in chemical compounds consisting of atoms of nonmetallic elements, there are pairs consisting of electrons with opposite spins, i.e., rotating around their nuclei in opposite directions. Since the movement of negatively charged particles in space leads to the formation of electronic clouds, which ultimately ends with their mutual overlapping. What are the consequences of this process for atoms and what does it lead to?
Physical properties of covalent bond
It turns out that between the centers of two interacting atoms a two-electron cloud arises with a high density. The electrostatic forces of attraction between the negatively charged cloud itself and the nuclei of atoms are amplified. A portion of energy is released and the distance between the atomic centers decreases. For example, at the beginning of the formation of the H 2 molecule, the distance between the nuclei of hydrogen atoms is 1.06 A, after the clouds overlap and the formation of a common electron pair is 0.74 A. Examples of covalent bonds formed by the above mechanism can be found among both simple and among complex inorganic substances. Its main distinguishing feature is the presence of common electron pairs. As a result, after the appearance of a covalent bond between atoms, for example, hydrogen, each of them acquires an electronic configuration of inert helium, and the resulting molecule has a stable structure.
Spatial form of the molecule
Another very important physical property of a covalent bond is directivity. It depends on the spatial configuration of the molecule of matter. For example, when two electrons overlap with a spherical cloud shape, the appearance of the molecule is linear (hydrogen chloride or hydrogen bromide). The shape of the water molecules, in which the s- and p-clouds are hybridized, is angular, and very strong particles of gaseous nitrogen have the form of a pyramid.
The structure of simple substances - non-metals
Having found out what connection is called covalent, what signs it has, now is the time to deal with its varieties. If atoms of the same non-metal — chlorine, nitrogen, oxygen, bromine, etc. — enter into interaction with each other, then the corresponding simple substances are formed. Their common electron pairs are located at the same distance from the centers of atoms, not shifting. For compounds with a non-polar type of covalent bond, the following features are inherent: low boiling and melting points, insolubility in water, dielectric properties. Next, we will find out for which substances a covalent bond is characteristic at which a shift of common electron pairs occurs.
Electronegativity and its effect on the type of chemical bond
The property of a certain element to attract electrons to itself from the atom of another element in chemistry is called electronegativity. The scale of values of this parameter proposed by L. Pauling can be found in all textbooks on inorganic and general chemistry. Its greatest value - 4.1 eV - is fluorine, the smaller is other active non-metals, and the lowest indicator is typical for alkali metals. If elements that differ in their electronegativity react with each other, then inevitably one, more active, will attract negatively charged particles of the atom of a more passive element to its core. Thus, the physical properties of the covalent bond directly depend on the ability of the elements to give electrons to the public. The resulting common pairs are no longer arranged symmetrically with respect to the nuclei, but are shifted towards the more active element.
Features of compounds with a polar bond
The substances in the molecules of which the joint electron pairs are asymmetric with respect to the nuclei of atoms include hydrogen halides, acids, chalcogen compounds with hydrogen and acid oxides. These are sulfate and nitrate acids, sulfur and phosphorus oxides, hydrogen sulfide, etc. For example, a hydrogen chloride molecule contains one common electron pair formed by unpaired electrons of hydrogen and chlorine. It is shifted closer to the center of the Cl atom, which is a more electronegative element. All substances with a polar bond in aqueous solutions dissociate into ions and conduct an electric current. Compounds having a polar covalent bond, the examples of which we have given, also have higher melting and boiling points compared to simple non-metal substances.
Methods for breaking chemical bonds
In organic chemistry, the substitution reactions of saturated hydrocarbons with halogens proceed by a radical mechanism. A mixture of methane and chlorine in the light and at ordinary temperature reacts in such a way that the chlorine molecules begin to split into particles carrying unpaired electrons. In other words, the destruction of the common electron pair and the formation of very active –Cl radicals are observed. They are capable of acting on methane molecules in such a way that they break the covalent bond between carbon and hydrogen atoms. An active particle –H is formed, and the free valency of the carbon atom takes on a chlorine radical, and chloromethane becomes the first reaction product. Such a mechanism for splitting molecules is called homolytic. If the total pair of electrons completely passes into the possession of one of the atoms, then they speak of a heterolytic mechanism, characteristic of reactions that take place in aqueous solutions. In this case, the polar water molecules will increase the rate of destruction of the chemical bonds of the dissolved compound.
Double and triple bonds
The vast majority of organic substances and some inorganic compounds contain not one, but several common electron pairs in their molecules. The multiplicity of covalent bonds reduces the distance between atoms and increases the stability of compounds. It is customary to talk about them as chemically resistant. For example, in a nitrogen molecule there are three pairs of electrons, they are indicated in the structural formula by three dashes and determine its strength. The simple substance, nitrogen, is chemically inert and can react with other compounds, for example, with hydrogen, oxygen or metals only when heated or under high pressure, as well as in the presence of catalysts.
Double and triple bonds are inherent in such classes of organic compounds as unsaturated diene hydrocarbons, as well as substances of a number of ethylene or acetylene. Multiple bonds determine the basic chemical properties: addition and polymerization reactions occurring at the points of their rupture.
In our article, we gave a general description of covalent bonds and considered its main types.