The physics of the structure of matter was first seriously studied by Joseph J. Thomson. However, many questions remained unanswered. After some time, E. Rutherford was able to formulate a model of the structure of the atom. In the article we will consider the experience that led him to the discovery. Since the structure of matter is one of the most interesting topics in physics lessons, we will analyze its key aspects. We will learn what the atom consists of; we will learn to find the number of electrons, protons, neutrons in it. Let's get acquainted with the concept of isotopes and ions.
Electron discovery
In 1897, the English scientist Joseph John Thomson (his portrait can be seen below) studied electric current, i.e., the directional movement of charges in gases. At that time, physics already knew about the molecular structure of matter. It was known that all bodies consist of matter, which consists of molecules, and the latter - of atoms.
Thomson found that under certain conditions, gas atoms emit particles with a negative charge (q el <0). They are called electrons. The atom is neutral, which means that if electrons fly out of it, then positive particles must also be contained there. What is a part of an atom with a plus sign? How does it interact with a negatively charged electron? What determines the mass of an atom? Another scientist was able to answer all these questions.
Rutherford Experience
The physicist already owned the initial information on the structure of matter in 1911. Ernest Rutherford discovered what we call today the atomic nucleus.
There are substances with a strange property: they spontaneously emit various particles, both positive and negative. Such substances are called radioactive. Rutherford called positively charged elements alpha particles (α particles).
They have a charge with a “+” sign, equal to two elementary (q α = + 2e). The weight of the elements is approximately equal to the four masses of the hydrogen atom. Rutherford took a radioactive drug that emits alpha particles, and they bombarded a thin film of gold (foil) with their stream.
He found that most α-elements almost do not change their direction, passing through metal atoms. But there are very few who lean back. Why is this happening? Knowing the physics of the structure of matter, we can answer: because inside the gold atoms, like any others, there are positive elements that repel alpha particles. But why does this happen only with a very small number of elements? Because the dimensions of the positively charged part of the atom are much smaller than itself. Rutherford came to this conclusion. He called the positively charged part of the atom the nucleus.
Atom device
Physics of the structure of matter: molecules are composed of atoms that contain a tiny positively charged part (core) surrounded by electrons. The neutrality of the atom is explained by the fact that the total negative charge of the electrons is equal to the positive - the nucleus. q core + q el = 0. Why do not the electrons fall on the nucleus, because they are attracted? To answer this question, Rutherford suggested that they rotate as planets move around the Sun and do not collide with it. It is the movement that allows this system to be stable. The atomic model invented by Rutherford was called planetary.
If the atom is neutral, and the number of electrons in it must be integer, then the charge of the nucleus is equal to this value with a plus sign. q kernels = + z * e. z is the number of electrons in a neutral atom. In this case, the total charge is zero. How to find out the number of electrons in an atom? You need to use the periodic system of elements. The size of the atom is about 10 -10 m. And the nucleus is 100 thousand times smaller - 10 -15 m.
Imagine that we increased the size of the core to 1 meter. In a solid, the distance between the atoms is approximately equal to the size of themselves, which means that the size will increase to 10 5 , and this is 100 km. That is, an atom practically consists of a void, which is why alpha particles mostly fly through the foil, almost without deviating.
Core structure
The physics of the structure of matter is such that the core consists of particles of two kinds. Some of them have a positive charge. If we consider an atom with three electrons, then inside it are three particles with a positive charge. They are called protons. Other elements do not have an electric charge - neutrons.
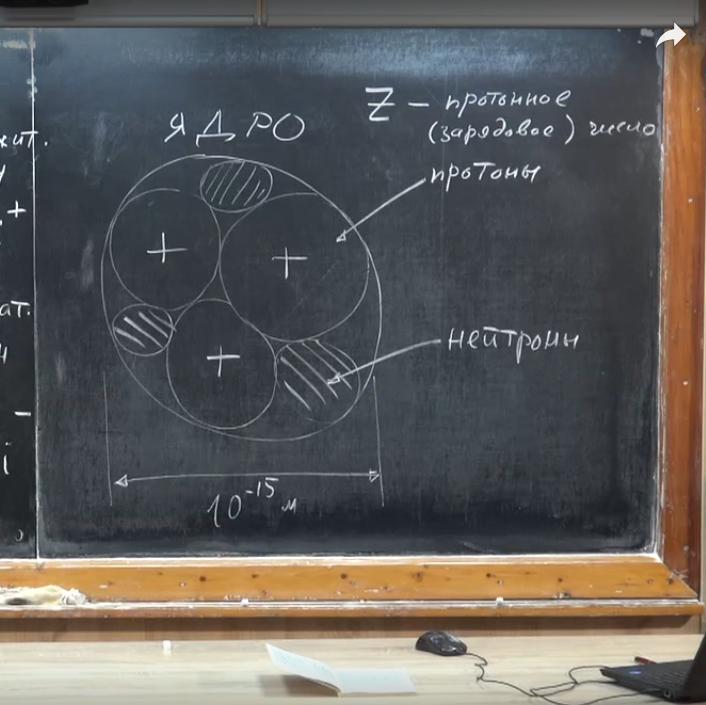
The masses of the proton and neutron are approximately equal. Both the one and the other particles have a weight much greater than the electron. m proton ≈ 1837m e . The same goes for the neutron mass. The conclusion follows from this: the weight of positively and neutrally charged particles is a factor that determines the mass of an atom. Protons and neutrons have a common name - nucleons. The weight of an atom is determined by their number, which is called the mass number of the nucleus. We designated the number of electrons in the atom by the letter z, but since it is neutral, the number of positive and negative particles must coincide. Therefore, z is also called the proton or charge number.
If the mass and charge numbers are known, then we can find the number of neutrons N. N = A - z. How to find out how many nucleons and protons are in the nucleus? It turns out that in the periodic table near each element a number is indicated, which chemists call the relative atomic mass.
If you round it, we get nothing more than the mass number or number of nucleons in the nucleus (A). The sequence number of the element is the number of protons (z). Knowing A and z, it is easy to find N - the number of neutrons. If the atom is neutral, then the number of electrons and protons is equal.
Isotopes
There are varieties of the nucleus in which the number of protons is the same, and the number of neutrons may differ (meaning the same chemical element). They are called isotopes. In nature, atoms of different varieties are mixed, so chemists measure the average mass. That is why the relative weight of an atom in the periodic table is always a fractional number. We will figure out what will happen to a neutral atom if we remove an electron from it or, conversely, place an extra one.
Jonah
Consider a neutral lithium atom. There is a nucleus, two electrons are located on one shell and three - on the other. If we take one of them, we get a positively charged core. q core = 3e. Electrons compensate only two of the three elementary charges, and we get a positive ion. It is denoted as follows: Li + . An ion is an atom in which the number of electrons is less than or greater than the number of protons in the nucleus. In the first case, it is a positive ion. If we add an extra electron, then there will be four of them, and we will get a negative ion (Li - ). Such is the physics of the structure of matter. So, a neutral atom differs from an ion in that in it the electrons completely compensate for the charge of the nucleus.